Effects of Synedrella nodiflora (L.) Gaertn. Leaves Extracted by Liquid-Liquid Partition on Germination and Growth of Pakchoi (Brassica chinensis Jusl var parachinensis (Bailey) Tsen & Lee)
Keywords:
Synedrella nodiflora (L.) Gaertn. , allelopathy, seed germination , osmotic potentialAbstract
Background and Objectives : Organic farming is one approach that benefits the quality of life of farmers, consumers, and the environment. However, effective weed management remains a necessary part of agricultural practice. Chemical herbicides are commonly used for this purpose, but they tend to accumulate in the environment and cause widespread negative impacts. In nature, allelopathy is a phenomenon whereby plants produce and release organic compounds into the environment that can inhibit the growth of neighboring plants. These naturally occurring organic compounds are usually biodegradable and thus have minimal potential for environmental accumulation. Synedrella nodiflora (L.) Gaertn. is a plant species that has been reported to exhibit allelopathic activity. However, prior studies have only evaluated its crude extracts, without further fractionation or identification of the specific chemical constituents responsible for the allelopathic effects. Therefore, this study aims to separate the extract into different fractions to determine which one holds allelopathic potential. The goal is to identify components that could be developed into environmentally safe herbicides that support sustainable agriculture and pose minimal risks to humans and ecosystems.
Methodology : Leaves of Synedrella nodiflora were extracted using 80% methanol by soaking for 24 hours, followed by filtration through filter paper. The resulting filtrate was evaporated to dryness using a rotary evaporator to obtain a crude extract. Half of this crude extract was subjected to liquid-liquid partitioning by adjusting the pH to 5 using sulfuric acid, then adding chloroform and gently shaking. Two layers formed: the chloroform phase (moderate polar extract) and the aqueous phase (aqueous acid extract). Half of the aqueous acid extract was further treated by adjusting the pH to 10 using ammonium hydroxide. A mixture of chloroform and methanol (3:1) was then added and gently shaken. The water-soluble fraction from this process was collected and termed the aqueous basic extract. Each extract was evaporated to dryness using a rotary evaporator at 39°C. The dried extracts were tested for their effects on seed germination and seedling growth of Chinese flowering cabbage (Brassica chinensis Jusl. var. parachinensis (Bailey) Tsen & Lee). Concentrations of 0–2 mg/mL were used, and observations were made over five days. Germination percentages were recorded and used to calculate IC50 values via probit analysis. The osmotic potential of the extracts was determined using the freezing point depression method, in which the solution was chilled in a salt-ice mixture and the lowest temperature and phase-change temperature were recorded. These values were used to calculate osmotic potential. To assess whether osmotic effects influenced germination, PEG solutions with equivalent osmotic potentials to those of the crude and moderate polar extracts were prepared and tested for seed germination effects. Measurements included shoot length, root length, fresh weight, and dry weight to evaluate the impact of the extracts on plant growth.
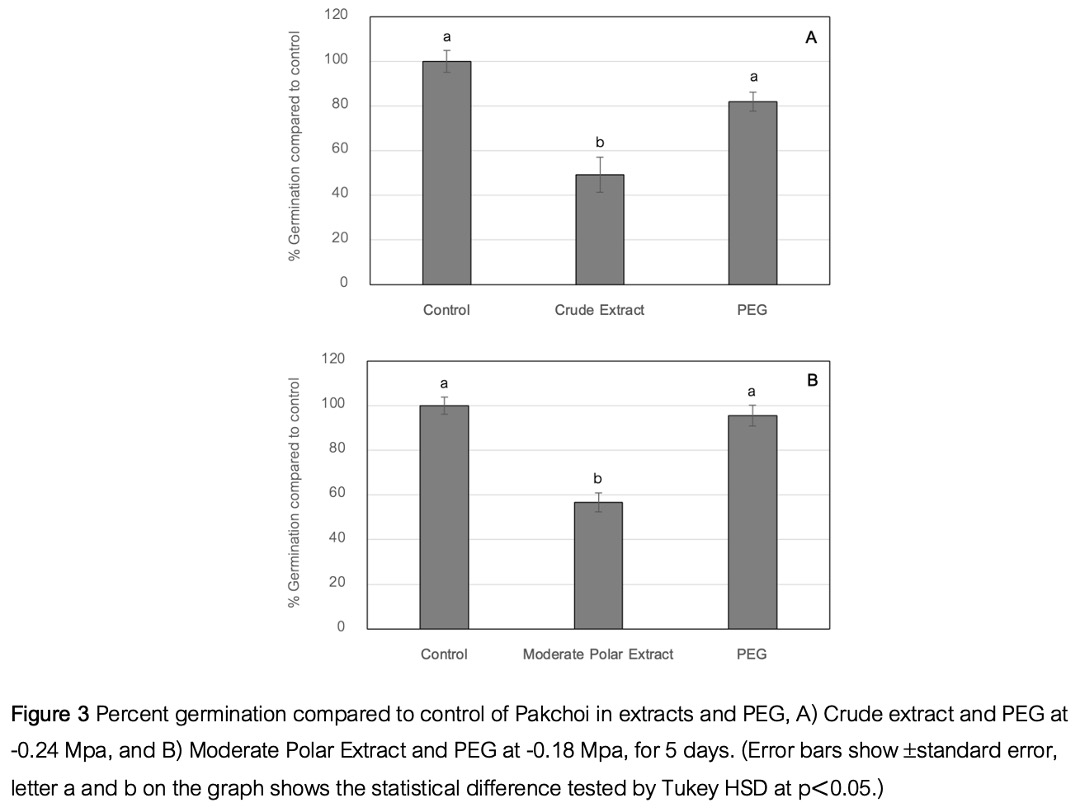
Main Results : The results showed that both the crude extract and moderate polar extract significantly inhibited seed germination compared to the control group (p < 0.05). Increasing the concentration of the extracts led to a greater reduction in germination percentage. In contrast, the aqueous acid and aqueous basic extracts did not result in statistically significant differences in seed germination compared to the control group (p > 0.05). The IC50 values for the crude and moderate polar extracts were found to be 0.94 mg/mL and 2.5 mg/mL, respectively. The osmotic potentials of these extracts at IC50 concentrations were -0.24 MPa and -0.18 MPa, respectively. When the effects of these extracts were compared to PEG solutions with equivalent osmotic potentials, it was found that the PEG solutions did not significantly affect seed germination (p > 0.05). However, the crude and moderate polar extracts at the same osmotic potential significantly reduced germination compared to the control group (p < 0.05), indicating that the inhibition was not due to osmotic effects. Furthermore, the crude and moderate polar extracts significantly inhibited shoot and root growth, and reduced fresh weight (p < 0.05). Shoot growth was inhibited by 100% and 94.3%, and root growth by 100% and 66.1%, respectively. Fresh weight was reduced by 63.5% and 69.5%, respectively. However, there were no significant effects on dry weight.
Conclusions : This study confirmed that crude and moderate polar extracts from Synedrella nodiflora can significantly inhibit seed germination and seedling growth of Chinese flowering cabbage, demonstrating clear allelopathic potential. Among the three extract fractions—moderate polar, aqueous acid, and aqueous basic—only the moderate polar extract exhibited consistent inhibitory effects. The aqueous acid and basic extracts did not show any clear allelopathic activity. The observed effects were not caused by osmotic potential, as demonstrated by the control PEG treatments. Therefore, it can be concluded that the moderate polar extract contains bioactive allelochemicals responsible for the observed inhibition. Further research is needed to identify the specific compounds in the moderate polar extract responsible for this allelopathic activity and assess their potential for development as natural herbicides for sustainable agricultural applications.
References
Ain, Q., Mushtaq, W., Shadab, M., & Siddiqui, M.B. (2023). Allelopathy: an alternative tool for sustainable agriculture. Physiology Molecular Biology Plants, 29, 495–511.
Azirak, S., & Karaman, S. (2008). Allelopathic effect of some essential oils and components on germination of weed species. Acta Agriculturae Scandinavica Section B-Soil and Plant Science, 58, 88-92.
Boyd N, & Van Acker, R. (2004). Seed germination of common weed species as affected by oxygen concentration, light, and osmotic potential. Weed Science, 589-596.
El-Gawad, A. A., Elshamy, A., El Gendy, A. E.-N., Gaara, A., & Assaeed, A. (2019). Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules, 24(3), 584. doi.org/10.3390/molecules24030584
Geddes, C.M., Cavalieri, A., Daayf, F., & Gulden, R.H. (2015). The allelopathic potential of Hairy Vetch (Vicia villosa Roth.) Mulch. American Journal of Plant Sciences, 6(16), 2651-2663.
Gxasheka, M., Mbita, Z., Laka, K., Mndela, M., & Dlamini, P. (2025) Phytochemical analysis and allelopathic potential of an aggressive Encroacher shrub, Euryops floribundus (Asteraceae). Plants (Basel), 14(4), 601. doi: 10.3390/plants14040601.
Harborne, J.B. (1998). Phytochemical methods: A guide to modern techniques of plant analysis (3rd Ed.), Bury St Edmunds: Chapman & Hall.
Hossain, M.K., Anwar, S., & Nandi, R. (2016). Allelopathic effects of Mikania cordata on forest and agricultural crops in Bangladesh. Journal of Forest Research, 27, 155–159.
Hossen, K., Das, K. R., Asato, Y., Teruya, T., & Kato-Noguchi, H. (2022). Allelopathic activity and characterization of allelopathic substances from Elaeocarpus floribundus Blume leaves for the development of bioherbicides. Agronomy, 12(1), 57. doi.org/10.3390/agronomy12010057
Hsiung, Y.C., Chen, Y.A., Chen, S.Y., Chi, W.C., Lee, R.H., & Chiang, T.Y. (2013). Volatilized myrcene inhibits growth and activates defense responses in rice roots. Acta Physiologie Plantarum, 35, 2475–2482.
John, D. A., & Babu, G. R. (2021). Lessons from the aftermaths of Green Revolution on food system and health. Frontiers in Sustainable Food Systems, 5, 644559. doi.org/10.3389/fsufs.2021.644559.
Kambire, D. A., Touré, K., Yapi, T. A., Paoli, M., Bighelli, A., Boti, J. B., & Tomi, F. (2024). Comparative study of the chemical composition of root, stem and leaf essential oils from Synedrella nodiflora (L.) Gaertn. Compounds, 4(3), 521-533.
Kato-Noguchi, H., Hamada, Y., Kojima, M., Kumagai, S., Iwasaki, A., & Suenaga, K. (2023). Allelopathic substances of Osmanthus spp. for developing sustainable agriculture. Plants, 12(2), 376. doi.org/10.3390/plants12020376
Kingthong, Y., & Phraprasert, P. (2023). Allelopathic effect from Synedrella nodiflora (L.) Gaertn.) on seed-germinating physiology of pakchoi (Brassica chinensis Jusl var parachinensis (Bailey) Tsen & Lee). Burapha Science Journal, 28(3), 1653-1670. Retrieved from https://scijournal.buu.ac.th/index.php/sci/article/view/4758 (in Thai)
La Iacona, M., Lombardo, S., Mauromicale, G., Scavo, A., & Pandino, G. (2024). Allelopathic Activity of three wild Mediterranean Asteraceae: Silybum marianum, Cynara cardunculus var. sylvestris, Galactites tomentosus. Agronomy, 14(3), 575. doi.org/10.3390/agronomy14030575.
Li, Z.-H., Wang, Q., Ruan, X., Pan, C.-D., & Jiang, D.-A. (2010). Phenolics and plant allelopathy. Molecules, 15(12), 8933-8952. doi.org/10.3390/molecules15128933
Lopez, M. L. Bonzani, N. E. ,& Zygadlo, J.A. (2008). Allelopathic potential of Tagetes minuta terpenes by a chemical, anatomical and phytotoxic approach. Biochemical Systematics and Ecology, 36(12), 882-890.
Nishida, N., Tamotsu, S., Nagata, N., Saito, C., & Sakai, A. (2005). Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. Journal Chemical Ecology, 31, 1187–1203.
Pace, R., & Benincasa, P. (2010). Effect of salinity and low osmotic potential on the germination and seedling growth of rapeseed cultivars with different stress tolerance. Italian Journal of Agronomy, 5, 69-77.
Salam, I.U., Ahmed, M., & Hussain, F. (2018). A study of different parameters of osmotic potential compared with weed (Chenopodium album) on wheat and chickpea crop. Pakistan Journal of Botany, 50(3): 963-967.
Scepanovic, M., Koscak, L., Sostarcic, V., Pismarovic, L., Milanovic-Litre, A., & Kljak, K. (2022). Selected phenolic acids inhibit the initial growth of Ambrosia artemisiifolia L. Biology, 11(4), 482. doi.org/10.3390/biology11040482
Scognamiglio, M, & Schneider, B. (2020). Identification of potential allelochemicals from donor plants and their synergistic effects on the metabolome of Aegilops geniculata. Frontier of Plant Science, 11, 1046. doi: 10.3389/fpls.2020.01046
Walne, C. H., Gaudin, A., Henry, W. B., & Reddy, K. R. (2020). In vitro seed germination response of corn hybrids to osmotic stress conditions. Agosystems, Geosciences & Environment, 3(1), e20087.
Wang, C., Qi, J., Liu, Q., Wang, Y., & Wang, H. (2022). Allelopathic potential of aqueous extracts from Fleagrass (Adenosma buchneroides Bonati) against two crop and three weed species. Agriculture, 12(8), 1103. doi.org/10.3390/agriculture12081103
William, C. S., & William, F.S. (1931). A method for the determination of the freezing point depression of aqueous solutions particularly those containing protein. Journal of Biological Chemistry, 91, 217-226.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



