Biological Development of the Fighting Beetle (Xylotrupes gideon) in a Confined Rearing System
Keywords:
fighting beetle , biology , rearing , larvaAbstract
Background and Objectives : The fighting beetle (Xylotrupes gideon) plays an important role in the ecosystem, contributing significantly to nutrient cycling and soil enrichment through the consumption of decaying plant matter during their larval stages. Furthermore, the adult beetle is a viable food source for humans, presenting an opportunity for sustainable food production. Populations in their natural habitats are currently declining due to several factors, including the destruction of natural habitats, which limits the beetles' ability to reproduce, pollution from agricultural practices involving chemical use and overharvesting for consumption. Although some aspects of this insect's biology were explored, the complete biology of this species, particularly that of the pre-adult stages, which reside underground, remains little known due to the difficulty of observation. This study aimed to investigate the complete life cycle and developmental biology of the fighting beetle from the egg stage to the reproductive adult stage, under controlled, confined rearing conditions, utilizing an artificial diet to mimic their natural food sources.
Methodology : Five breeding pairs of healthy males and females were used for offspring production. Subsequently, mated females were allowed to oviposit in a 25×22 cm culture pot filled with a fermented sawdust artificial diet. After allowing for 5 days for oviposition, eggs were verified via careful examination. Ten eggs were carefully selected from a single female and were meticulously monitored under a stereomicroscope to analyze the developmental processes occurring before the larval stage. The majority of the eggs obtained from all five female beetles were incubated until they successfully hatched into first instar larva (L1). Subsequently, 100 larvae (L1) were individually reared in 32-ounce plastic cups until they developed into the reproductive adult stage. Throughout the developmental period, data on the number of eggs per female, larval morphology at each instar, body weight, developmental duration, survival rate, sex ratio, and adult lifespan were recorded.
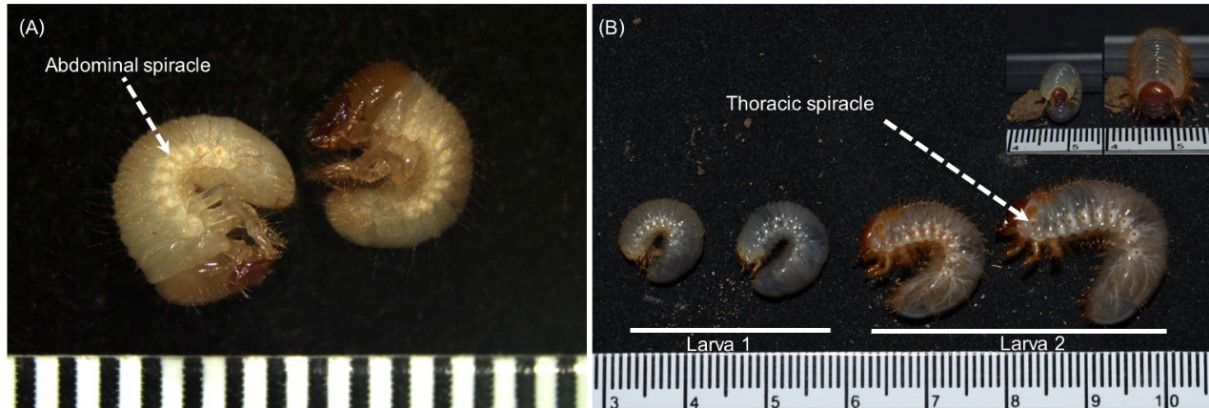
Main Results : The study demonstrated that the fighting beetle can be successfully reared in a confined rearing system using a formulated artificial diet that mimics their natural food source, allowing them to develop into reproductive adults. The average number of eggs laid per female was 34.20 ± 12.19. The eggs were white, oval-shaped, and approximately 0.3 cm in length. Fertilized eggs exhibited progressive developmental changes as observed through stereomicroscopy. Observation of eggs collected 5 days after oviposition revealed a translucent appearance. By 10 days post-female oviposition, the eggs had increased in size, and an opaque spot was observed, indicating embryonic development. By 15 days post-female oviposition, larval development was observed with clear segmentation of the body. By 25 days post-female oviposition, significant development was apparent with pigmentation of the body and clearly distinguishable head and mandible structures. These eggs hatched into L1 larvae after approximately 25-30 days. Newly hatched L1 showed a white head and body with reddish-brown hair on the body. The head color transitioned to brown over time. Larval development progressed through molting, with larvae molting from L1 to L2 and L2 to L3, with each stage approximately 30 days. The L3 larval stage took approximately 180 days. At the late L3 stage, the larva made a pupation chamber and their transparent skin changed to yellow and wrinkled before developing into a pupa stage. The durations from the pupa-teneral adult, teneral adult-reproductive adult and reproductive adult-natural death stages were 21.38±2.26, 32.82±3.24 and 53.81±15.59 days, respectively. The survival rates (%) from L1-L2, L2-L3, L3-pupa, pupa-teneral adult, and teneral adult-reproductive adult stages were 100, 92, 82, 82, and 71%, respectively. Forming a pupation chamber by L3 larva is a prominent behavior, providing an open space for pupal and adult development. The pupation chamber of this species was oval-shaped with rounded ends and possessed thick and rigid walls. This study yielded 41 reproductive adult males and 30 reproductive adult females, resulting in a male-to-female sex ratio of 1.00:0.73. A chi-square test indicated that this ratio was not statistically significant. Males exhibited variations in horn length. The coloration of adult fighting beetles from this study was classified into two primary groups: reddish-brown and black. Reddish-brown was the predominant color observed in the reared beetles. Within the reddish-brown group, there were varying shades that didn’t form distinct subgroups.
Conclusions : This study successfully reared the fighting beetle, elucidating their biology from the egg to the reproductive adult stages, under confined condition and using an artificial diet. The diet was formulated with rubber sawdust, dried cow manure, soil, wheat flour and water, mixed in specific proportions. The 71% survival rate of the fighting beetle from the larval stage to the reproductive adult stage under solitary rearing conditions (limited space) indicates that the formulated diet is adequate for supporting their development to the reproductive adult stage. This study provides critical insights into the feasibility and optimization of large-scale rearing for various applications, including sustainable conservation, commercial sale and alternative protein production from edible insect.
References
Abenis, K.O., Lit, I.L., Jr., Caasi-Lit, M.T., & Naredo, J.C.B. (2018). First record of the dynastid beetle, Xylotrupes gideon L., on field corn. The Philippine Entomologist, 32(2), 147-150.
Ali, N., Tabia, A.N.M., Zakila, F.A., Fauzaia, W.N.F.M., & Hassana, O. (2013) Yield performance and biological efficiency of empty fruit bunch (EFB) and palm pressed fibre (PPF) as substrates for the cultivation of Pleurotus Ostreatus. Jurnal Teknologi (Sciences & Engineering), 64(1), 93-99.
Alvarez, H.J.G. (2011). Beetle breeding and its applicability in conservation. Scarabs, 59, 1-5.
Ando, T., & Niimi, T. (2019). Development and evolution of color patterns in ladybird beetles: A case study in Harmonia axyridis. Development Growth & Differentiation, 61, 73-84.
Bedford, G.O. (1980). Biology, ecology and control of palm Rhinoceros beetles. Annual Review of Entomology, 25, 309-339.
Chung, M.Y., Kwon, E.Y., Hwang, J.S., Goo, T.W., & Yun, E.Y. (2013). Establishment of food processing methods for larvae of Allomyrina dichotoma, Korean horn beetle. Journal of Life Science, 23(3), 426-431.
Ek-Amnuay, P. (2009). Breeding and rearing beetles (1st edition). Bangkok: Amarin printing and publishing public co. Ltd. (in Thai)
EL-Zohairy, M.M., EL-Deeb, M.A., Hashem, H.H., & EL-Sayed Arafa, O. (2009). Biological studies on Oryctes rhinoceros L. (Coleoptera: Scarabaeidae). Egyptian Journal of Agricultural Research., 87(2), 395-402.
Emlen, D.J., Hunt, J., & Simmons, L.W. (2005). Evolution of sexual dimorphism and male dimorphism in the expression of beetle horns: phylogenetic evidence for modularity, evolutionary lability, and constraint. The American Naturalist, 116 (Supplement): S42-68.
Food intelligence center. (2022). Thailand's edible insect food industry in 2022. Retrieved from https://fic.nfi.or.th/upload/info_graphic/pdf81.pdf. (in Thai)
Gautier, M., Yamaguchi, J., Foucaud, J., Loiseau, A., Ausset, A., Facon, B., Gschloessl, B., Lagnel, J., Loire, E., Parrinello, H., Severac, D., Donnadieu, C., Manno, M., Berges, H., & Gharbi, K. (2018). The genomic basis of color pattern polymorphism in the harlequin ladybird. Current Biology, 28, 3296-3302.
House, C.M., Simmons, L.W., Kotiaho, J.S., Tomkins, J.L., & Hunt, J. (2010). Sex ratio bias in the dung beetle Onthophagus taurus: adaptive allocation or sex-specific offspring mortality?. Evolutionary Ecology. 1-10.
Inkrod, C., Raita, M., & Laosiripojana, N. (2017). Characteristics of lignin extracted from pararubber wood sawdust via organosolv fractionation. Journal of Sustainable Energy & Environment, 8, 71-76.
Kojima, W., Nakakura, T., Fukuda, A., Lin, C.P., Harada, M., Hashimoto, Y., Kawachi, A., Suhama, S., & Yamamoto, R. (2020). Latitudinal cline of larval growth rate and its proximate mechanisms in a rhinoceros beetle. Functional Ecology, 34, 1577-1588.
Komariah, A., Tatara, R.A., & Bustami, D.A. (2016). Efficacy of rhinoceros beetle (Xylotrupes gideon) nano chitosan and calcium mouthwash in reducing quantity oral cavity bacteria among elementary school age children. International Journal of Advanced Biological and Biomedical Research, 4(3), 238-245.
Krongdang, S., Phokasem, P., Venkatachalam, K., & Narin Charoenphun, N. (2023). Edible Insects in Thailand: An overview of status, properties, processing, and utilization in the food industry. Foods, 12, 2-24.
Lachowsky, L.E., & Reid, M.L. (2014). Developmental mortality increases sex-ratio bias of a size-dimorphic bark beetle. Ecological Entomology, 39, 300-308.
Lai, J. (2012). Captive breeding of Dynastes hercules paschoali. Scarabs, 69, 1-9.
Li, S., Yu, X., & Feng, Q. (2019). Fat body biology in the last decade. Annual Review of Entomology, 13(10), 1-19.
Lim, J.R., Lee, S.S., Lee, E.J., Kim, W., & Choi, C.H. (2023). Study on the suitable fermentation period of berry sawdust for the development of Protaetia brevitarsis larva. Journal of Applied Entomology, 62(3), 183-191.
Pimentel, C.S., & Ayres, M.P. (2020). Competition and climate affect body size and sexual size dimorphism in pine sawyer beetles. Bulletin of Insectology, 73(2), 265-273.
Preacher K.J. (2001). Calculation for the chi-square test: An interactive calculation tool for chi-square tests of goodness of fit and independence. [Computer software] Retrieved from https://quantpsy.org/chisq/chisq.htm.
Rennesson, S. (2019). Wrestling beetles and ecological wisdom: How insects contribute to the cosmopolitics of Northern Thailand. Southeast Asian Studies, 8(1), 3-24.
Roff D.A. (1992). The evolution of life histories, theory and analysis. Chapman and Hall. New York. USA.
Sanchez, M.V., Krause, J.M., González, M.G., & Dinghi, P.A. (2010). The pupation chamber of dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 64(3), 277-284.
Shukla, A., Panchal, H., Mishra, M., Patel, P. R., Srivastava, H.S., Patel, P., & Shukla, A.K. (2014). Soil moisture estimation using gravimetric technique and FDR probe technique: A comparative analysis. American International Journal of Research in Formal, Applied & Natural Sciences, 8(1), 89-92.
Souza, T.B.D., Albuquerque, L.S.C.D., Luciana Iannuzzi, L., Fábio Correia Costa, F.C., Marc Gibernau, M., & Maia, A.C.D. (2023). Egg development and viability in three species of Cyclocephala (Coleoptera: Scarabaeidae: Dynastinae). Bulletin of Entomological Research, 113(1), 118-125.
Ukoroije, R.B., & Bobmanuel, R.B. (2019). A call for domestication and mass rearing of Oryctes owariensis larvae (Coleoptera: Scarabaeidae). Noble International Journal of Scientific Research, 3(10), 103-108.
Ukoroije, R.B., & Bawo, D.D.S. (2019a). Studies on the life cycle of Oryctes owariensis beauvis growth under laboratory condition in Wilberforce Island, Nigeria. Ekiti State University Journal of Science and Technology (EJST), 4(1), 43-49.
Ukoroije, R.B., & Bawo, D.D.S. (2019b). Morphological studies of the life Stages of Oryctes owariensis Beauvois (Coleoptera: Dynastinae: Scarabaeidae). SSRG International Journal of Agriculture & Environmental Science (SSRG-IJAES), 6(4), 24-27.
Yoon, C.H., Jeon, S.H., Ha, Y.J., Kim, S.W., Bang, W.Y., Bang, K.H., Gal, S.W., Kim, I.S., & Cho, Y.S. (2020). Functional chemical components in Protaetia brevitarsis Larvae: Impact of supplementary feeds. Food Science of Animal Resources, 40(3), 461-473.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



