Synthesis of 4-Methoxycinnamyl Alcohol and Its Derivatives via Lithium Aluminium Hydride : Investigation of Reduction Product Formation by qNMR Analysis
Keywords:
4-methoxycinnamic acid , 4-methoxycinnamyl alcohol , qNMR, reduction reaction , lithium aluminium hydrideAbstract
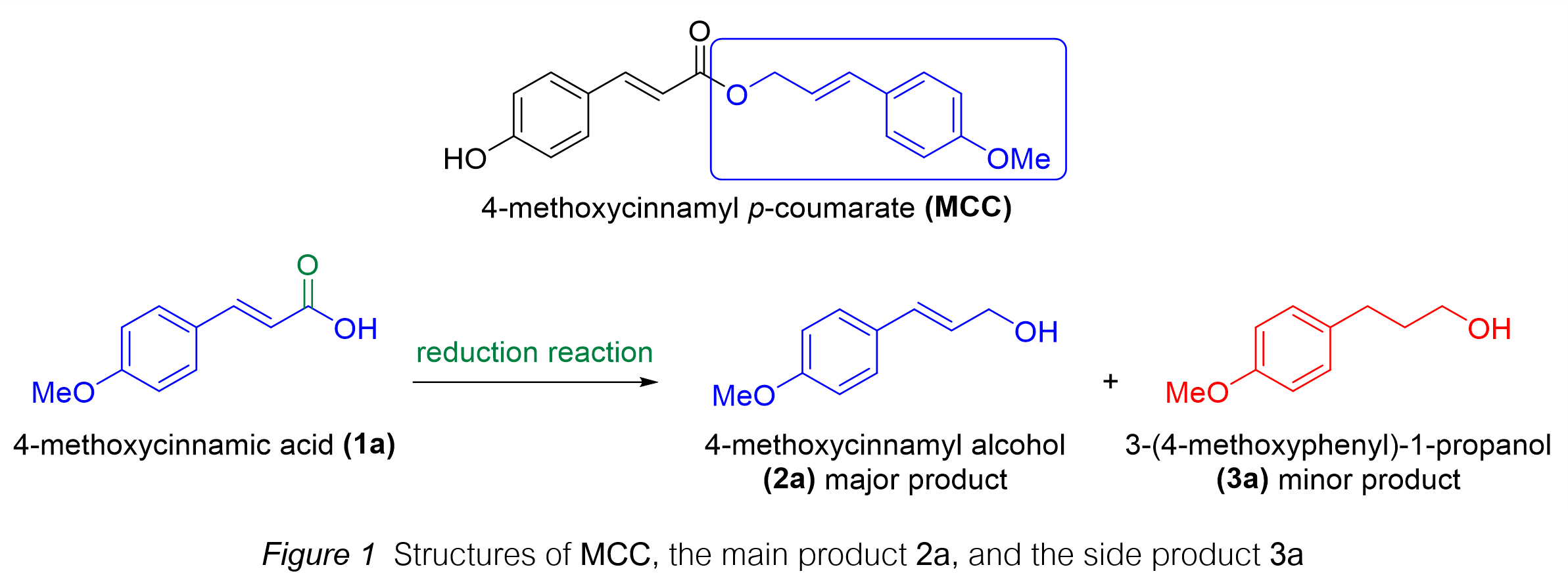
Background and Objectives : Natural products can be obtained through synthesis, either to increase quantity or improve quality. 4-methoxycinnamyl alcohol, found in Etlingera pavieana (Pierre ex Gagnep.) R.M.Sm. and Foeniculum vulgare Mill, is the target compound for synthesis in this research. It serves as a precursor for synthesizing 4-methoxycinnamyl p-coumarate (MCC), a compound with interesting bioactive properties in pharmaceuticals and cosmetics. These properties include anti-inflammatory, antioxidant, and tyrosinase inhibitory effects, making MCC a potential candidate for drug and cosmetic applications. However, a challenge encountered in synthesizing 4-methoxycinnamyl alcohol via the reduction of 4-methoxycinnamic acid with lithium aluminum hydride is the formation of side products due to the reduction of the double bond, leading to a lower yield of the main product and difficulty in purification. This issue arises because lithium aluminum hydride is a strong reducing agent that tends to react with both the carboxyl group and the conjugated double bond, leading to a mixture of reduced products. Therefore, this study aims to investigate reaction conditions to minimize side product formation and to synthesize derivatives of 4-methoxycinnamyl alcohol under optimal conditions. The study also explores the influence of different substituents on the reaction outcome. The product yields were analyzed using quantitative Nuclear Magnetic Resonance (qNMR) with acetanilide as an internal standard.
Methodology: The study began with optimizing the conditions for synthesizing 4-methoxycinnamyl alcohol by controlling key reaction factors, including lithium aluminum hydride quantity, reaction time, and temperature. The initial amount of lithium aluminum hydride was set at 0.5 equiv., increasing in increments of 0.5 equiv. The reaction was conducted under an argon atmosphere at room temperature to 0°C for 4 to 24 hours. Once the optimal conditions were identified, they were applied to synthesizing derivatives of 4-methoxycinnamyl alcohol by modifying the para-substituent groups, including hydrogen (H) from cinnamic acid, hydroxy group (OH) from coumaric acid, and O-tetrahydropyranyl group (OTHP) from 4-(tetrahydro-2H-pyran-2-yloxy) cinnamic acid. These derivatives were selected to examine the effects of electronic and steric factors on the reaction pathway. Finally, the yields of both the main and side products were analyzed using qNMR, with acetanilide as an internal standard.
Main Results: The experimental results revealed that increasing the amount of lithium aluminum hydride at the same reaction time and temperature led to a higher formation of side products. This suggests that excess lithium aluminum hydride promotes non-selective reduction, affecting both the carboxyl and double bond functionalities. Therefore, reaction time was increased while keeping lithium aluminum hydride quantity constant at 0.2 equiv. at room temperature to observe the reaction trend. It was found that significant side product formation persisted, indicating that temperature might be a more influential factor in controlling selectivity. Subsequently, lowering the temperature to 0°C while maintaining the lithium aluminum hydride amount at 0.2 equiv. for 4 hours resulted in a higher yield of the main product. This confirms that temperature plays a crucial role in directing the reduction pathway. Increasing the lithium aluminum hydride quantity to 3.0 equiv. further improved the main product yield, likely due to enhanced reduction efficiency at the carboxyl site. However, extending the reaction time to 8 hours did not significantly change the yield, suggesting that the reaction reaches equilibrium within 4 hours. Therefore, the optimal conditions for synthesis were determined to be 3.0 equiv. of lithium aluminum hydride at 0°C for 4 hours, balancing high yield and selectivity. However, when these conditions were applied to synthesizing derivatives of 4-methoxycinnamyl alcohol, the yields of the main products remained low to moderate, with minor side product formation. This was attributed to the resonance and steric effects of electron-donating groups and structural factors of the compounds. The presence of hydroxyl groups, for example, influenced the electronic environment, potentially altering the reduction pathway. Similarly, the bulky tetrahydropyranyl group affected steric accessibility, leading to different product distributions.
Conclusions: The synthesis of compound 4-methoxycinnamyl alcohol via reduction under an argon atmosphere using lithium aluminum hydride showed that lowering the temperature reduced double bond reduction but did not significantly enhance selectivity toward the carboxyl group. Increasing the quantity of lithium aluminum hydride and reaction time improved carboxyl reduction and overall yield. However, excessive reaction time could lead to consecutive reactions or degradation of the starting material, highlighting the importance of balancing reaction parameters. The most suitable conditions for synthesis were 3.0 equiv. of lithium aluminum hydride at 0°C for 4 hours, yielding a moderate amount of the main product with minimal side products. Nevertheless, these conditions were not optimal for synthesizing derivatives of 4-methoxycinnamyl alcohol due to the impact of structure and substituent groups on yield. Further investigation in this study, such as the use of alternative reducing agents or protective group strategies, may enhance the yield of the main product and minimize side product formation. This research provides valuable insights into the influence of reaction conditions on reduction selectivity, which can be applied to similar synthetic processes in natural product and pharmaceutical chemistry.
References
Amin, N. , & T. Claridge (2017). Quantitative NMR Spectroscopy. Oxford: Oxford University Press.
Carey, F. A., & Sundberg, R. J. (2007). Advanced organic chemistry: Part B: Reactions and synthesis (5th ed.). Springer.
Hu, L. H., Zou, H. B., Gong, J. X., Li, H. B., Yang, L. X., Cheng, W., Zhou, C. X., Bai, H., Guéritte, F., & Zhao, Y. (2005). Synthesis and biological evaluation of a natural ester sintenin and its synthetic analogues. Journal of Natural Products, 67(10), 1681–1687.
Lall, N., Kishore, N., Binneman, B., Twilley, D., van de Venter, M., du Plessis-Stoman, D., Boukes, G., & Hussein, A. (2015). Cytotoxicity of syringin and 4-methoxycinnamyl alcohol isolated from Foeniculum vulgare on selected human cell lines. Natural product research, 29(18), 1752-1756.
Malz, F., & Jancke, H. (2005). Validation of quantitative NMR. Journal of pharmaceutical and biomedical analysis, 38(5), 813-823.
Smith, M. B., & March, J. (2007). March’s advanced organic chemistry: Reactions, mechanisms, and structure (6th ed.). Wiley.
Srisook, E., Palachot, M., Mankhong, S., & Srisook, K. (2017). Anti-inflammatory effect of Etlingera pavieana (Pierre ex Gagnep.) R.M.Sm. Rhizomal extract and its phenolic compounds in lipopolysaccharide-stimulated macrophages. Pharmacognosy magazine, 13(Suppl 2), S230.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



