Determination of Mercury (II) Ion Using Rhodamine 6 G Hydrazide on Natural Polymers Derivative Solid Support
Keywords:
mercury (II) ion , rhodamine 6 G hydrazideAbstract
Background and Objectives : Mercury (II) ions (Hg2+) are highly toxic metal ions, and even minor contamination in water sources can adversely affect living organisms. When the body is exposed, permanent damage is done to the genetic material, nervous system, and brain. The World Health Organization (WHO) has established that the concentration of inorganic mercury in drinking water should not exceed 29.9 nanomolar. Therefore, the development of a detection kit for mercury (II) ions is crucial. In this study, a detection kit for Hg2+ ions in the form of an environmentally friendly film was developed. The film was prepared by incorporating Rhodamine 6G hydrazide (L6G) into a cellulose triacetate (CTA) matrix, a cellulose derivative, with tris(2-ethylhexyl) phosphate (TEHP) as a plasticizer. Previous reports have indicated that L6G molecules exhibit high selectivity toward Hg2+ ion in a 10% tetrahydrofuran (THF) solution. However, this solvent limitation prevents L6G from detecting Hg2+ ion in aqueous samples. It was hypothesized that the L6G-based film developed in this study could be used for the detection of Hg2+ ions in water samples.
Methodology : The L6G film was prepared using the casting method by mixing Rhodamine 6G hydrazide (L6G) with cellulose triacetate (CTA) with tris(2-ethylhexyl)phosphate (TEHP) serving as a plasticizer. The selectivity of the film for metal ion detection was investigated by immersing the prepared film in solutions containing various metal ions. The film intensity was measured using Image J program. Additionally, the optimal conditions for film preparation were studied, including the appropriate amounts of CTA, TEHP, and ligand L6G. The optimal conditions for using the film as a detection kit were also examined, including the appropriate detection time, the effect of pH and the concentration range of mercury (II) ion interacting with the L6G film. Moreover, the detection limit and limit of quantification of film L6G towards mercury (II) ion were also studied.
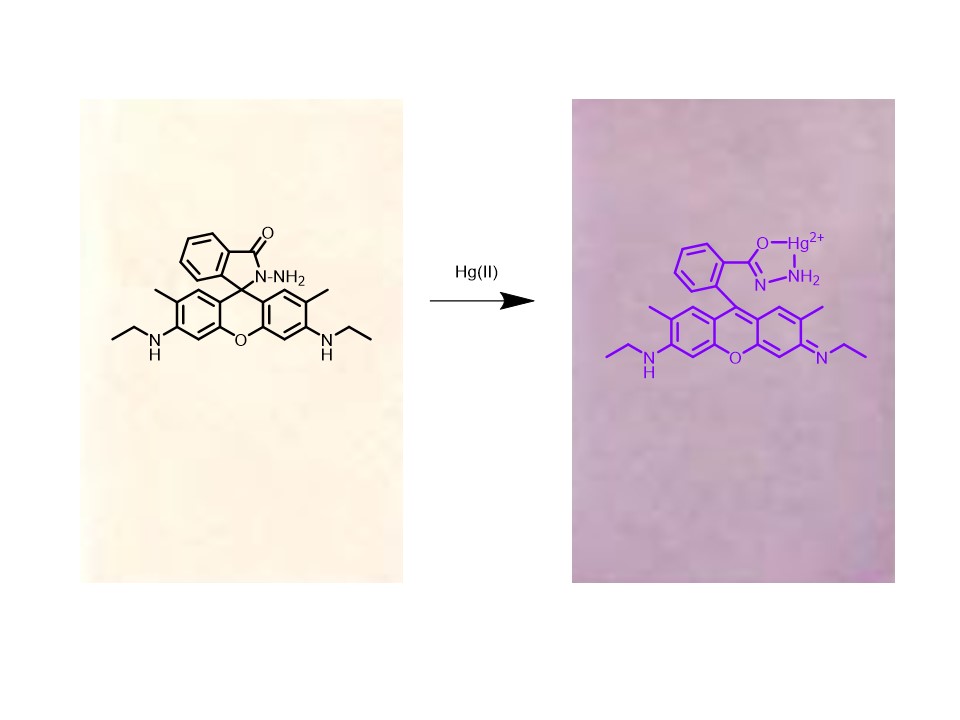
Main Results : The prepared L6G film was observed to have a smooth, pale pink appearance and to be water-insoluble under the studied optimal conditions. The prepared film was tested for its ability to selectively detect metal ions in water, and it was determined that L6G film exhibited high specificity for Hg2+ ions. This was evidenced by a color change of the film from pale pink to purple, which occurred exclusively in the presence of Hg2+ ions. This color change resulted from the coordination covalent bonding between Hg2+ ions and the L6G ligand within the film. As a consequence of electron donation from oxygen and nitrogen atoms in the L6G ligand, a color change from pale pink to purple was observed in the film due to spirolactam ring opening. The optimal conditions for preparing the L6G film were investigated. It was found that the most suitable amount of CTA was 0.2 grams, as this quantity produced a transparent film with an appropriate thickness. If the amount of CTA is too low, a thin film will be obtained, whereas an excessive amount of CTA results in a thick film. The TEHP content was identified as being optimal at 0.05 grams, as any excess led to the formation of a rigid, matte film, which obstructed water penetration. The L6G ligand amount was best at 6.0 milligrams, yielding the highest color intensity, whereas excessive L6G led to an opaque and non-uniform film Based on the study of optimal conditions for detecting Hg2+ ions in aqueous solutions, the L6G film was found to require an ideal immersion time of 180 minutes at a pH of 5.0. The detection was conducted at 0 minutes, immediately after the film was removed from the solution, dried, and its color intensity measured. The concentration range of Hg2+ ions that exhibited the strongest linear correlation with film color intensity was found to be 0 – 10 ppm, with an R² value of 0.9984. The detection limit and the minimum measurable concentration of mercury (II) ions using the L6G film were measured at 0.51 ppm and 1.66 ppm, respectively.
Conclusions : In this research, an optical chemical sensor in the form of a film was developed for the detection of Hg2+ ions dissolved in water. The sensor molecule, rhodamine 6G hydrazide (L6G), was incorporated into cellulose triacetate (CTA) using the casting method, with THEP serving as a plasticizer. The prepared detection film was found to be a thin, transparent, pale pink sheet that was water-insoluble and stable in aqueous environments. Based on the selectivity study of the prepared film for sensing metal ions, the film was observed to exhibit high specificity toward Hg2+ ions in which the film’s color changing from pale pink to purple, whereas other metal ions did not interfere with the sensing process. Therefore, the capability of the developed colorimetric detection kit to detect Hg2+ ions in aqueous solutions has been demonstrated. One of the benefits of preparing the film using this method is its simplicity, as it does not require expensive tools or equipment.
References
Feng, L., Sha, J., Chen, Y., Liu, B., Zhang, H., & Lu, C. (2015). Conjugated polymer and spirolactam rhodamine-B derivative co-functionalized mesoporous silica nanoparticles as the scaffold for the FRET-based ratiometric sensing of mercury (II) ions. Microporous and Mesoporous Materials, 208, 113.
Ghaedi, M., Ahmadi, F., & Shokrollahi, A. (2007). Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry Journal of Hazardous Materials, 142, 272.
Iyer, D. K., Shaji, A., Singh, S. P., Tripathi, A., Hazra, A., Mandal, S., & Ghosh, P. (2023). A review on rhodamine probes for metal ion recognition with a future on artificial intelligence and machine learning. Coordination Chemistry Reviews, 495(15), 215371.
Leermakers, M., Baeyens, W., Quevauviller, O., & Horvat, M. (2005). Mercury in environmental samples: Speciation, artifacts and validation. TrAC Trends in Analytical Chemistry, 24(5), 383.
Mahajan, R.K., Kaur, I., & Lobana, T.S. (2003). A mercury(II) ion-selective electrode based on neutral salicylaldehyde thiosemicarbazone. Talanta, 59, 101.
Morsi, R. E., Elsawy, M., Manet, I., & Ventura, B. (2020). Cellulose acetate fabrics loaded with rhodamine B hydrazide for optical detection of Cu(II). Molecules, 25, 3751.
O’Meara, J.M., Brjesson, J. , & Chettle, D.R. (2000). Improving the in vivo X-ray fluorescence (XRF) measurement of renal mercury. Applied Radiation and Isotopes, 53, 639.
Rompo, M., Watchasit, S., Imyim, A., & Suksai, C. (2018). Development of nanofibers containing rhodamine B as colorimetric Chemosensor for Cu2+ determination. Burapha Science Journal, 23, 1094.
Sahan, T., Erol, F., & Yilmaz, S. (2018). Mercury(II) adsorption by a novel adsorbent mercapto-modified bentonite using ICP-OES and use of response surface methodology for optimization. Microchmical Journal, 38, 360.
Tuzen, M., Karaman, I., Citak, D., & Soylak, M. (2009). Mercury(II) and methyl mercury determinations in water and fish samples by using solid phase extraction and cold vapour atomic absorption spectrometry combination. Food and Chemical Toxicology, 47, 1648.
Yang, Z. (2024). Voltammetry for quantitative determination of trace mercury ions in water via acetylene black modified carbon paste electrode. Alexandria Engineering Journal, 87, 107.
Yang, Y.-K., Yook, K.-J., & Tae, J. (2005). A Rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. Journal of the American Chemical Society, 127, 46760.
Zhang, Y., Cao, X., Zhen, L., & Wang, X. (2021). A mesoporous silica-based fluorescent chemosensor bearing bis-Schiff base for the sensitive detection of Cu2+ ions. Journal of Solid State Chemistry, 297, 122093.
Zhang , Z., Han, R., Chen, S., Zheng, F., Ma, X., Pan, M., & Wang, S. (2023). Fluorescent and colorimetric dual-mode strategy based on rhodamine 6G hydrazide for qualitative and quantitative detection of Hg2+ in seafoods. Foods, 12, 1085.
Zhu, W., Tang, J., & Huang, L. (2020). Ultra-sensitive electrochemical determination of mercury ions based on the dithizone modified electrode. International journal of electrochemical science, 15 (1), 168-176.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



