Optimization for Lutein Extract and Antioxidant Activity from Marigold Flowers (Tagetes erecta L.) Using Ultrasonic Assisted Extraction
Keywords:
marigold, lutein, antioxidant activity, optimization, ultrasonic extractionAbstract
Background and Objectives : Marigold (Tagetes erecta L.) is an economically valuable flowering plant that can be cultivated year-round. It is recognized as a rich natural source of lutein, a carotenoid compound that plays a significant role in reducing the risk of age-related macular degeneration. Moreover, lutein exhibits antioxidant properties that help protect cells from oxidative damage, potentially reducing the risk of chronic diseases. However, since the human body cannot synthesize lutein, it must be obtained through dietary intake. Conventional methods of lutein extraction typically rely on organic solvents such as hexane and acetone. Although effective, these solvents are hazardous to both human health and the environment. Therefore, this study aims to develop a safer and more sustainable method for extracting lutein and antioxidant compounds from marigold petals. The proposed method utilizes ultrasound-assisted extraction (UAE) combined with ethanol, a food-grade solvent known for its environmental friendliness and low toxicity. The research specifically focuses on identifying optimal extraction conditions by evaluating three critical parameters: ultrasonic amplitude, extraction time, and ethanol concentration. The goal is to maximize extraction efficiency while preserving the bioactive properties of the target compounds.
Methodology : Fresh marigold flowers were first washed three times with reverse osmosis (RO) water to remove impurities and potential contaminants. The flowers were then air-dried in the shade to reduce initial moisture levels. Once adequately dried, the petals were separated from the calyx and subjected to freeze-drying at -40 °C under vacuum pressure not exceeding 10 Pa until the moisture content dropped below 10%. The dried marigold petals were ground into a fine powder using a high-speed multifunctional dry grinder operating at 25,000 rpm. The powder was then mixed with ethanol in varying concentrations and subjected to ultrasound-assisted extraction using a specialized ultrasonic device. The experimental design was based on the Box-Behnken Design (BBD), a response surface methodology that allows for the assessment of multiple variables and their interactions. The study investigated three independent variables: ultrasonic amplitude (20%, 40%, and 60%), extraction time (5, 15, and 25 minutes), and ethanol concentration (40%, 60%, and 80%). The extracted samples were analyzed for lutein content using High-Performance Liquid Chromatography (HPLC) and for antioxidant activity using the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay. Data from the experiments were subjected to Analysis of Variance (ANOVA) using the SPSS software to identify statistically significant factors and interactions affecting the extraction efficiency.
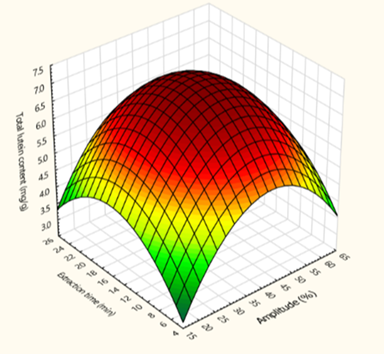
Main Results : The study revealed that both ethanol concentration and extraction time significantly influenced the efficiency of lutein and antioxidant extraction. Ethanol, being a semi-polar solvent, has the ability to solubilize both polar and non-polar bioactive compounds. This characteristic enables it to co-extract phenolic compounds and flavonoids, which are known to be present alongside lutein in marigold petals. These compounds collectively contribute to the overall antioxidant capacity of the extract. Increasing the extraction time up to an optimal level facilitated better mass transfer and diffusion of bioactive compounds from plant tissues into the solvent. However, prolonged extraction times beyond the optimal range led to increased internal system temperatures, which may promote oxidative degradation, especially in heat-sensitive compounds like lutein. This degradation ultimately results in lower yields. Regarding ultrasonic amplitude, the study found that higher amplitudes generally enhanced the release of lutein from plant tissues. This is attributed to the cavitation effect generated by ultrasonic waves, where microbubbles collapse violently, causing the rupture of plant cell walls and facilitating the release of intracellular compounds. Nevertheless, excessive amplitude may lead to elevated pressures and temperatures within the extraction medium, which could again result in the degradation of sensitive compounds. Interestingly, while amplitude had a noticeable effect on lutein yield, it did not significantly influence the antioxidant activity of the extracts. This could be due to the insufficient intensity of the ultrasonic treatment to disrupt cellular structures enough to release additional antioxidant compounds beyond those already extracted by ethanol.
Conclusions : Marigold flowers are a valuable botanical source of lutein and antioxidant compounds, both of which can be effectively extracted using ultrasound-assisted extraction techniques. Based on response surface methodology analysis, the optimal extraction conditions were determined to be 42% amplitude, 16 minutes of extraction time, and 80% ethanol concentration. Under these optimized conditions, the maximum lutein yield was 7.06 mg per gram of sample, and the highest antioxidant activity observed was 79.82%. The ANOVA results demonstrated a high degree of model fit and prediction reliability, with R² values of 0.9691 for lutein content (Y1) and 0.9943 for antioxidant activity (Y2). Furthermore, the Significance F values were found to be less than 0.001, indicating the statistical significance and robustness of the predictive models developed through the Box-Behnken Design. This study underscores the potential of ultrasound-assisted extraction using ethanol as a green, efficient, and scalable technique for extracting valuable bioactive compounds from marigold flowers. Not only does it offer a safer alternative to traditional organic solvents, but it also supports sustainability goals by minimizing environmental impact and reducing extraction time and energy consumption.
References
Abia, S., Chanes, J., & Cano, M. (2022). Effect of Ultrasound-Assisted Extraction of Carotenoids from Papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils. Molecules, 27, 638
Altemimi, A., Lightfoot, D., Kinsel, M., & Watson, D. (2015). Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach. Molecules, 20, 6611-6625.
Derrien, M., Badr, A., Gosselin, A., Desjardins, Y., & Angers, P. (2017). Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). Food Science and Technology, 79, 170-177.
Hemwimol, S., Pavasant, P., & Shotipruk, A. (2006). Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrasonics Sonochemistry, 13, 543–548.
Ibrahim, A., & Sriherfyna, F. (2015). Effect of Temperature and Extraction Time on Physicochemical Properties of
Red Ginger (Zingiber officinale var. Rubrum) Extract with The Additional of Honey Combination as Sweetener for Functional Drink. Volume 3, 530-541.
Jaeschke, D., Rech, R., Marczak, L., & Mercali, G. (2017). Ultrasound as an alternative technology to extract carotenoids and lipids from Heterochlorella luteoviridis. Bioresource Technology, 224, 753-757.
Janepinich, P., Satirapipathkul, C., Kasetsomboon, N., & Yongphet, P. (2023). Enhancing lutein extraction from marigolds through ultrasound‑assisted optimization using response surface methodology. Maejo International Journal of Energy and Environmental Communication, 5(1), 14–19.
Kashyap, P., Singh, S., Singh, M., Gupta, A., Tandon, S., Shanker, K., Verma, R., & Verma, R. (2022). An efficient process for the extraction of lutein and chemical characterization of other organic volatiles from marigold (Tagetes erecta L.) flower, Food Chemistry, 396, 133647.
Kurniawan, J., Yusuf, M., Azmi, S., Salim, K., Prihastyanti, M., Indrawati, R., Heriyanto., Shioi, Y., Limantara, L., & Brotosudarmo, T. (2019). Effect of drying treatments on the contents of lutein and zeaxanthin in orange- and yellow-cultivars of marigold flower and its application for lutein ester encapsulation. In Proceeding Conference Series: Materials Science and Engineering. 509.
Manzoor, S., Rashid, R., Panda, B., & Sharma, V. (2022). Green extraction of lutein from marigold flower petals, process optimization and its potential to improve the oxidative stability of sunflower oil, Ultrasonics Sonochemistry, 85, 105994
Narkprasom, N., Narkprasom, K., & Upara, U. (2015). Optimization of Total Phenolic from Cleistocalyx nervosum by Microwave-Assisted Extraction. American Journal of Engineering and Applied Sciences, 8(3), 302-309.
Nguyen, D., Thrimawithana, T., Piva, T., Grando, D., & Huynh, T., (2023). Benefits of plant carotenoids against age-related macular degeneration. Journal of Functional Foods, 106.
Pingret, D., Tixier, A., & Chemat, F. (2013). Degradation during application of ultrasound in food processing: A review. Food Control, 31, 593-606.
Pratumyam, P., Lairungruang, K., Thimkorn, P., Obhasi, S., & Sangvichien, S. (2023). Antioxidant Activities and Total Phenolic Contents of Plook Fai Thatu Remedy. Journal of Traditional Thai Medical Research, 9 (1), 13-27. (in Thai)
Shimada, K., Fujikawa, K., Yahara, K., & Nakamura T. (1992). Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. Agr. Food Chem, 40, 945–948.
Ye, J., Feng, L., Xiong, J., & Xiong, Y. (2011). Ultrasound-assisted extraction of corn carotenoids in ethanol. Food Science and Technology, 46, 2131-2136.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



