The Optimal Harvesting Frequency for Production on a Number of Calanoid Copepod Parvocalanus crassirostris
Keywords:
optimal, harvesting frequency , calanoid copepod Parvocalanus crassirostrisAbstract
Background and Objectives : Parvocalanus crassirostris is a calanoid copepod species of significant ecological and nutritional importance, widely recognized as a premium live feed for the larviculture of marine ornamental fish. Its naupliar stage is particularly small, highly digestible, and enriched with essential polyunsaturated fatty acids (PUFAs), including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which play critical roles in enhancing larval growth, pigmentation, neural development, and survival rates. These nutritional qualities make P. crassirostris an optimal live diet for marine fish species with small mouth openings, such as butterflyfish and angelfish, which are otherwise challenging to rear. Distributed across tropical and subtropical waters—including coastal areas of Thailand and the Hawaiian Islands—this species has been the focus of intensive research aimed at improving culture techniques, optimizing production efficiency, and meeting the increasing demand for high-quality live feeds in aquaculture hatcheries.Despite its high potential, the continuous and efficient production of P. crassirostris remains challenging, mainly due to the delicate balance required between harvesting and population stability. Over-harvesting can severely deplete populations, while insufficient harvesting may lead to overcrowding, increased competition for resources, and reduced reproductive output. Previous studies have highlighted the importance of establishing optimal harvest intervals to maintain sustainable yields and prevent fluctuations in population dynamics. This study aimed to determine the most appropriate harvesting frequency for P. crassirostris, focusing on naupliar and copepodite stages, to develop guidelines applicable to commercial-scale production systems and ornamental fish hatcheries.
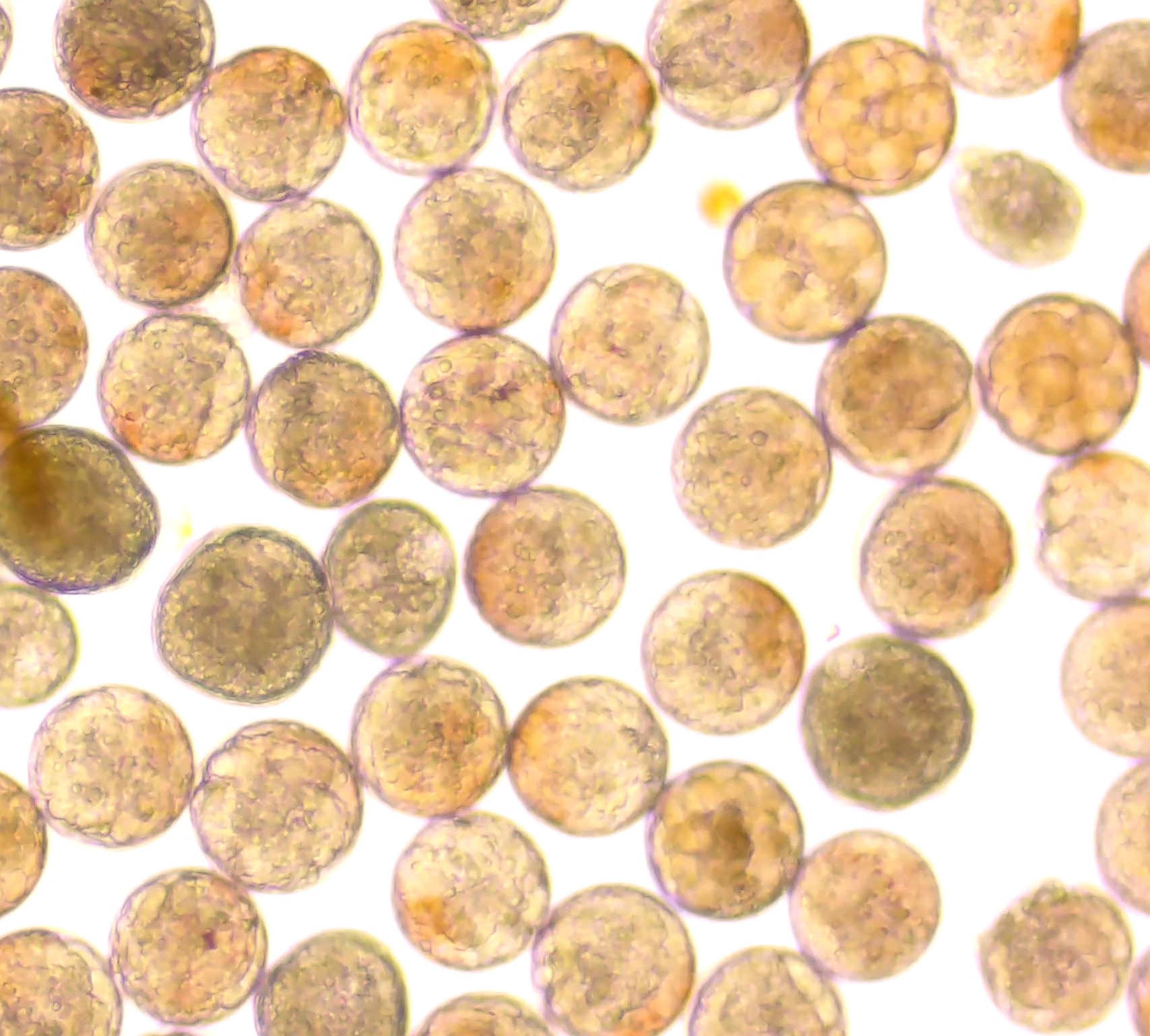
Methodology : The experiment was designed using a completely randomized design (CRD). Four different harvesting strategies were tested: non-harvest (control), harvesting once every other day, harvesting once every third day, and harvesting once every fifth day. Each harvest involved the removal of 20% of the culture volume, after which the tank was replenished with filtered, sterilized seawater. Twelve units of 5-litre glass tanks were divided into 4 triplicate treatments, 3 replicates each. Copepods were sourced from stock cultures maintained at the Cha-am Research Station, Institute of Marine Science, Burapha University, and were acclimated under controlled laboratory conditions. The initial stocking density was 1.99±0.13 ind./ml-1. In the current study, Cultures were fed daily with two microalgal species, including Isochrysis galbana & Chaetocerop sp. each at a concentration of 75,000 cells/ml-1 to 1 time per day. The culture was maintained at room temperature, salinity of 28 ppt, and a photoperiod of 24:0 h light:dark cycle. Routine culture management included siphoning bottom sediments and performing 20% water exchanges every three days to maintain optimal water quality and reduce organic waste buildup. Population density, specific growth rate (SGR), and the proportion of developmental stages (nauplii, copepodite, and adult) were monitored every two days over a 21-day experimental period. Sampling was conducted by collecting 49 mL of water from each tank, which was preserved with 10% neutral buffered formalin prior to counting under a stereo microscope (Olympus SZ).
Main Results : The results showed that the optimal harvest frequency was every other day, yielding a total number of 6,170 copepods (60% of the production). Harvesting once every third day had an average copepod output of 2,527.78 (25%). Harvesting once every fifth day gave the lowest total output (1,511.11 copepods, 15%). In compared were consisting of the harvesting frequencies in this experiment there was no significant effect on copepod density during the production (P > 0.05). The results showed that responded copepods by increasing the maximum copepod density was (mean±SE) 3.33±0.33, 4.53±0.82, 3.43±0.23, 3.22±0.39 ind./mL-1 at 4-6 daysbefore increasing density to below 1 ind./mL-1(mean±SE) 0.31±0.08, 0.36±0.09, 0.33±0.07, 0.24±0.04 ind./mL-1 at 14 days, respectively. P. crassirostris showed no significant (P>0.05) in the specific population growth rate (mean±SE) 0.06±0.125, 0.14±0.14, 0.21±0.11, 0.002±0.14 day-1. The proportion of copepods in adult stage (36.19%, 59.04%, 71.76% and 28.11% respectively), copepodite (9.57%, 20.16%, 14.51% and 13.11% respectively), and nauplii (54.24%, 20.28%, 13.76% and 58.78% respectively) did not show statistical differences between treatments. These results suggest that harvesting frequency does not disrupt age structure or recruitment dynamics within the population, provided that harvesting is conducted in moderate volumes.
Conclusions : Harvesting P. crassirostris every other day proved to be the most effective strategy for maximizing yield while maintaining population stability. This schedule ensures regular availability of nauplii and copepodites at peak nutritional value, which is essential for larval fish culture. Less frequent harvesting (every three or five days) resulted in lower yields and less efficient population turnover. These findings agree with previous studies on calanoid copepods, which highlighted that frequent but moderate harvesting supports culture performance, reduces density-related stress, and promotes reproduction.Harvesting every other day provides an optimal balance between sustainable biomass production and stable population dynamics, making it suitable for hatcheries requiring a steady supply of high-quality live feed. Non-harvest strategies may be useful for maintaining broodstock populations for future scaling. Moreover, this study underscores the potential of semi-recirculating or intensive culture systems to further enhance production efficiency, reduce operational costs, and meet the growing demand for sustainable aquaculture feeds. The insights gained here offer practical guidelines for improving P. crassirostris culture, ensuring reliable, cost-effective live feed supply chains for marine ornamental fish hatcheries and other aquaculture industries.
References
Alajmi, F., Zeng, C., & Jerry, D. (2015). Domestication as a Novel Approach for Improving the Cultivation of Calanoid Copepods: A Case Study with Parvocalanus crassirostris. PLoS ONE, 10, e0133269. doi:10.1371/journal.pone.0133269
Amerian Public Health Association, American Water Work Association, and Water Environment Federation. (1995). Standard method for the examination of water and wastewater. 19th ed. APHA, Washington, D.C.
Bendif, E.M., Probert, I., Schroeder, D. C., & de Vargas, C. (2013). On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J Appl Phycol, 25, 1763–1776.
Boxshall, G.A., & Halsey, S.H. (2004). An introduction to copepod diversity. Ray Society, Andover, U.K.
Burgess, A., & Callan, C. (2018). Effects of supplemental wild zooplankton on prey preference, mouth gape, osteological development and survival in first feeding cultured larval yellow tang (Zebrasoma flavescens). Aquaculture, 495, doi:10.1016/j.aquaculture.2018.06.046
Gamiao, S., Maria Haws, C., Tim, G., Chatham, C., & Carla. (2021). Survival in Parvocalanus crassiorostris cultures treated with commercial probiotic. In a thesis submitted to the graduate division of the University of Hawai’i at Holo in partial fulfillment of the requirements for the degree of master of science, 58 p.
Guillard, R. R. L., & Ryther, J. H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8(2), 229–239. doi.org/10.1139/m62-029.
Helm, M. M., & Laing, I. (1987). Preliminary observations on the nutritional value of ‘Tahiti Isochrysis’ to bivalve larvae. Aquaculture, 62, 281–288.
Humes, A.G., (1994). How many copepods? Hydrobiologia, 292–293, 1–7.
Kline, D. M., Chatham, K. C., & Charles, W. L. (2014). Advances in intensive copepod production technology. Global Aquaculture advocate. Retrieved from https://www.aquaculturealliance.org/advocate/advances-in-intensive-copepod-production-technology/?headlessPrint=AAAAAPIA9c8r7gs82oWZBA
Kline, M. D., & Laidley, C. W. (2015). Development of intensive copepod culture technology for Parvocalanus crassirostris: Optimizing adult density. Aquaculture, 435, 128-136.
Laidley, C. W., Burnell, A. F., Shields, R. J., Molnar, A., & Kotani, T. (2004). Marine ornamentals: captive culture progress at the Oceanic Institute. Global Aquaculture Advocate, 7, 53-54.
Lawson, T. J., & Grice, G. D. (1973). The developmental stages of Paracalanus crassirostris Dahl, 1894 (Copepoda, Calanoida). Crustaceana, 24(1), 43-56.
Liao, I.C., K.H. Su , & J.H. Lin, 1983. Larval foods for Penaeid prawn. In: CRC Handbook of Mariculture. Vol-1. Crustacean Aquaculture (J.P. McVey ed.). CRC press, Florida. pp. 43-69.
Liu, C. H., & Lin, H. J. (2001). Growth and fatty acid composition of the tropical marine haptophyte Isochrysis galbana and its related species. Aquaculture, 201(1–2), 135–150. doi.org/10.1016/S0044-8486(01)00552-2
McKinnon, A., Duggan, S., Nichols, P.D., Rimmer, M.A., Semmens, G.L., & Robino, B. (2003).The potential of tropical paracalanid copepods as live feeds in aquaculture. Aquaculture, 223, 89–106.
Okauchi M, Kawamura K, & Mizukami Y. (1997). Nutritive value of ‘Tahiti Isochrysis’ Isochrysis sp. for larval greasy back shrimp, Metapenaeus ensis. Bull Natl Res Inst Aquacult, 26, 1–11.
Payne, M.F., Rippingale, R.J., & Cleary, J.J. (2001). Cultured copepods as food for West Australian dhufish (Glaucosoma hebraicum) and pink snapper (Pagrus auratus) larvae. Aquaculture, 194, 137–150.
Payne, M.F., & Rippingale, R.J. (2001). Intensive cultivation of the calanoid copepod Gladioferens imparipes. Aquaculture , 201, 329–342.
Santhosh B., Anil M. K., Muhammed Anzeer F., Aneesh K. S., Mijo V. Abraham, Gopakumar G., Rani Mary George, Gopalakrishnan A. , & Unnikrishnan C. (2018). Culture Techniques of marine Copepods. CMFRI Booklet No.9/2018. ISBN 978-93-82263-23-4. Indian Council of Agricultural Research Central Marine Fisheries Research Institute, 142 p.
Shields, R. J., & Laidley, C. W. (2003). Intensively cultured paracalanid copepods: High quality diet for small tropical marine fish larvae? Global Aquaculture Advocate, 6(6), 80 p.
Shields, R.J., Kotani, T., Molnar, A., Marion, K., Kobashigawa, J., & Tang, L. (2005). Intensive cultivation of a subtropical paracalanid copepod, Parvocalanus sp., as prey for small marine fish larvae. Copepods Aquac, 209–223.
Solorzano, L. (1969). Determination of ammonia in natural water by the phenol hypochlorite method 1 1 This research was fully supported by U.S Atomic Energy Commission contract no. ATS (11-1) Gen 10, P.A. 20. Limnology and Oceanography, 14(5), 799-801. doi:10.4319/LO.1969.14.5.0799
Støttrup, J.J. (2000). The elusive copepods: their production and suitability in marine aquaculture. Aquac. Res, 31, 709–711.
Strickland, J.D.H., & Parsons, T.R. (1977). A Practical Handbook of Seawater Analysis. Minister of Supply and Services, Canada.
Valencia, T, G. A., Merino, G. E., Prieto-Guevara, M. J., Acosta Portillo, J. E., López Arboleda, J. E., & Chapman, F. A. (2022). Spawning Parvocalanus crassirostris at a high adult density: Explaining low adult population numbers and means for improving their intensive culture. Aquaculture, 546, 737347. doi.org/10.1016/j.aquaculture.2021.737347
van der Meeren, T., Olsen, R. E., Hamre, K., & Fyhn, H. J. (2008). Biochemical composition of copepods for evaluation of feed quality in production of juvenile marine fish. Aquaculture, 274 (2–4), 375–397. doi.org/10.1016/j.aquaculture.2007.11.041
Williamson, A., Blandon, I., Scarpa, J., Vega, R., & Siccardi, A. (2020). Copepod propagation and use as a live food for fish larviculture. Journal of the World Aquaculture Society, 51(4), 789–812. doi.org/10.1111/jwas.12697
Wiradana, P., Anjani, S., Yusup, D., Wiryatno, J., Melianawati, R., Nege, A., & Soegianto, A. (2020). Copepod growth populations (Acartia sp.) in outdoor mass culture tanks: Exploring natural feed potentials for sustainable aquaculture. Ecology, Environment and Conservation, 26, 2020-1383.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



