Differential Pulse Voltammetric Determination of Sulfite in Fruit Juice Samples Using an Unmodified Screen-Printed Graphene Electrode
Keywords:
sulfite, fruit juice beverage, screen printed electrode, electrochemical technique, portable deviceAbstract
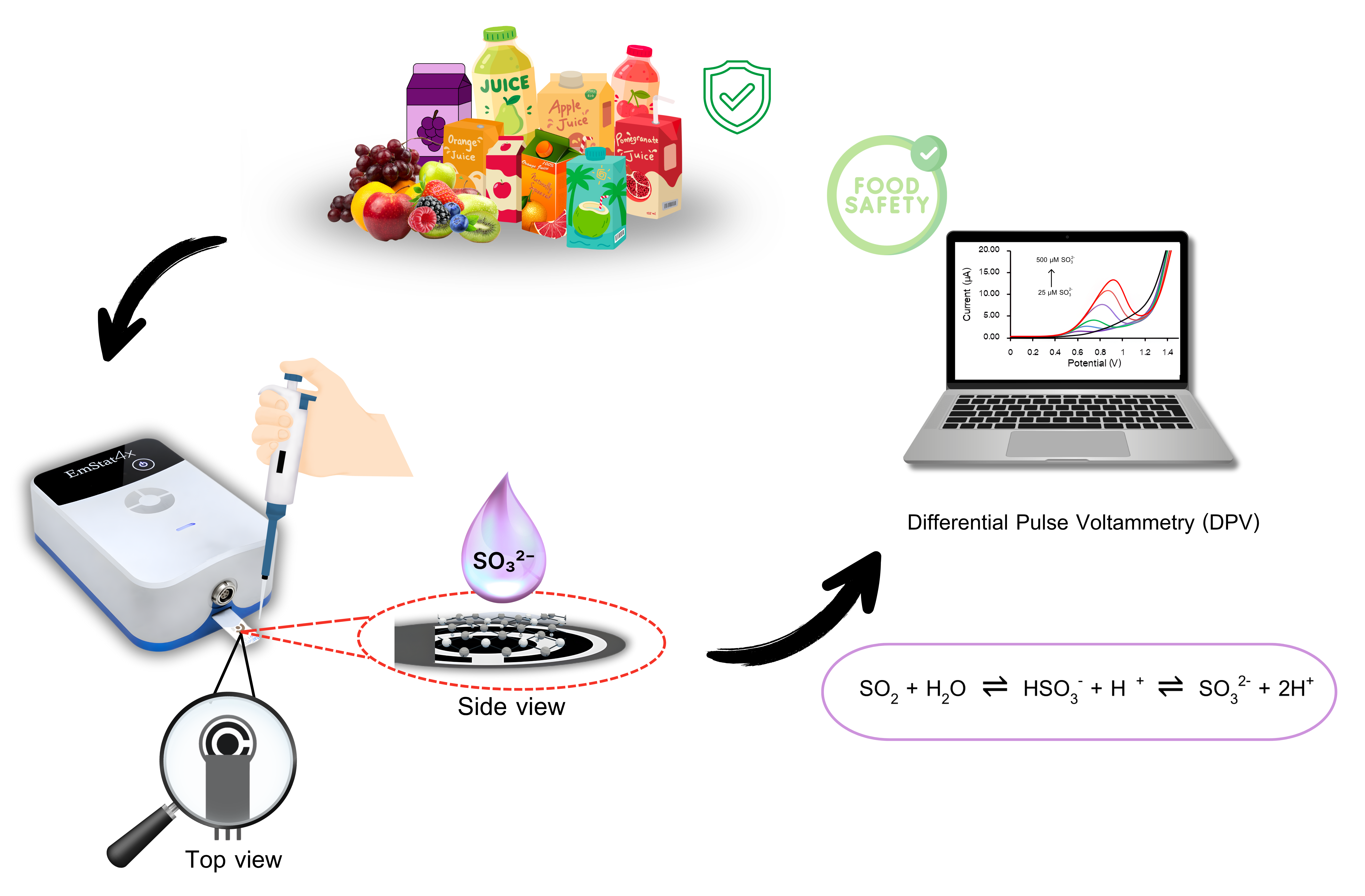
Background and Objectives : In recent years, consumers have become increasingly health-conscious and tend to consume fruit juices more frequently due to their valuable content of essential vitamins and minerals. However, commercially packaged fruit juices often contain food additives such as sulfites, which serve as preservatives to extend shelf life and maintain product quality. However, excessive intake of sulfites can pose potential health risks. Therefore, the development of analytical methods that are rapid, accurate and cost-effective for sulfite determination is essential. Electrochemical techniques, particularly Differential Pulse Voltammetry (DPV) combined with Screen-Printed Electrodes (SPEs) have gained considerable attention due to their high analytical sensitivity, low cost, minimal sample preparation requirements and suitable on-site analysis. In this study, an unmodified Screen-Printed Graphene Electrode (SPGE) was employed owing to its outstanding electrical conductivity, chemical stability and mechanical strength including robust structural properties. The objective of this research was to develop and apply the unmodified SPGE coupled with DPV for the determination of sulfite in commercially available fruit juice samples, as well as to evaluate the analytical performance in terms of sensitivity, accuracy and precision including feasibility of this method for practical field applications in the future.
Methodology : The sulfite analysis was carried out using DPV under optimized conditions. Standard sulfite solutions with concentrations ranging from 25 to 500 µM were prepared, and 80 µL of each solution was used for measurement. The applied potential was set between 0.00 and 1.50 V with a step potential of 20 mV, modulation amplitude of 80 mV, modulation time of 50 ms and interval time of 200 ms. The electrochemical behavior of the electrode was examined by Cyclic voltammetry (CV), while the optimal analytical conditions were determined by studying the effects of buffer pH and various DPV parameters, including step potential, modulation amplitude, modulation time and interval time considering the current response and peak characteristics. Analytical performance was evaluated in terms of linearity, limit of detection (LOD) and limit of quantitation (LOQ). Additionally, the precision of the method was assessed through repeatability and reproducibility tests in terms of relative standard deviation (%RSD). The study of interferences was investigated using compounds commonly present in fruit juices for instant ascorbic acid, citric acid, potassium sorbate and sucrose. For real sample analysis, nine (9) types of fruit juice such as coconut, apple, mixed fruit and vegetable, orange, lychee, longan, guava, strawberry and pandan were purchased from a local supermarket. All samples were analyzed using the unmodified screen-printed graphene electrode under the optimized DPV conditions.
Main Results : The screen-printed graphene electrode (SPGE) exhibited a significantly higher current response compared to the screen-printed carbon electrode (SPCE) indicating superior electrical conductivity as previously reported for graphene-based materials. This observation was consistent with the electrochemical impedance spectroscopy (EIS) results, which showed that the SPGE possessed a lower charge transfer resistance. The effect of pH on the voltametric response behavior of sulfite was also examined. The results revealed that the current increased within the pH range of 2.0 – 3.5 and decreased at pH values above 5.5. Therefore, pH 3.5 was selected as the optimal condition for subsequent measurements. For differential pulse voltametric parameters, the sharpest and most stable peak was obtained under the following conditions of step potential of 20 mV, a modulation amplitude of 80 mV, a modulation time of 50 ms and an interval time of 200 ms. These parameters provided a well-defined peak with minimal background noise, ensuring reliable quantitative analysis. Under the optimized conditions, the developed method demonstrated a linear response for sulfite determination within the concentration range of 25 – 500 µM with a correlation coefficient (r²) of 0.9945, indicating excellent linearity. The limits of detection (LOD) and quantification (LOQ) were found to be 12.27 µM and 40.92 µM, respectively. Interference studies revealed that sucrose caused the least interference, whereas ascorbic acid produced the most noticeable interference due to its electroactive nature. Nevertheless, the magnitude of this interference remained within an acceptable range and did not compromise the overall analytical performance. Application of the method to nine commercially available fruit juice samples yielded recovery values ranging from 80 – 120%, which fall within the acceptable limits for standard analytical procedures (AOAC standard). These results confirm that the proposed method provides satisfactory accuracy and precision. Overall, the findings indicate that the developed method is suitable for sulfite determination in fruit juice beverages and gives potential for practical implementation in quality control laboratories.
Conclusions : This study successfully developed a method for the rapid and efficient determination of sulfite in fruit juice using DPV coupled with an unmodified screen-printed graphene electrode (SPGE), achieving optimal results at an electrolyte pH of 3.5. The method demonstrated high analytical performance, exhibiting a wide linear range of 25 – 500 µM (r2 = 0.9945), low limits of detection and quantification of 12.27 µM and 40.92 µM, respectively and excellent precision with repeatability and reproducibility values of 6.04% and 5.68%, respectively. Furthermore, the method proved highly reliable when tested with real fruit juice samples, %recovery in the range of 80 – 120%, thereby confirming its accuracy, precision and stability for practical quality control applications.
References
AOAC, I. (2016). Guidelines for Standard Method Performance Requirements (Appendix F), Official Methods of Analysis. Retrieved from https://www.aoac.org/
Arnold, B. C., Machado, E. d. S., Martins, J. B. L., Paterno, L. G., & Politi, J. R. d. S. (2025). Exploring the Electronic Structure of Graphene and Graphene Ultrathin Films with Adsorbed Lithium. The Journal of Physical Chemistry C, 129(16), 7879-7893. doi.org/10.1021/acs.jpcc.4c08241
Asangil, D., Hudai Tasdemir, I., & Kilic, E. (2012). Adsorptive stripping voltammetric methods for determination of aripiprazole. J Pharm Anal, 2(3), 193-199. doi.org/10.1016/j.jpha.2012.01.009
Candido da Silva, M. C., Cardoso Viana, A., Araujo Carvalho, A. J. B., Colombo Pimentel, T., Magnani, M., & Dos Santos Lima, M. (2024). Impact of sulfite use and acidification on chemical quality components in thermally processed watermelon juices. Food Res Int, 180, 114088. doi.org/10.1016/j.foodres.2024.114088
Codex Alimentarius, C. (2019). General Standard for Food Additives (CODEX STAN 192-1995). Retrieved from Retrieved from https://www.fao.org/gsfaonline/docs/CXS_192e.pdf
Ehsan, M. A., Khan, S. A., & Rehman, A. (2021). Screen-Printed Graphene/Carbon Electrodes on Paper Substrates as Impedance Sensors for Detection of Coronavirus in Nasopharyngeal Fluid Samples. Diagnostics (Basel), 11(6). doi.org/10.3390/diagnostics11061030
Gutema, K. F., Mekonnen, M. L., Yilma, B. T., Asrat, T. E., Dellith, J., Diegel, M., Csaki, A., & Fritzsche, W. (2025). Rapid Colorimetric Detection of Sulfite in Red Wine Using Alginate-Copper Laccase Nanozyme with Smartphone as an Optical Readout. ACS Meas Sci Au, 5(1), 145-154. doi.org/10.1021/acsmeasuresciau.4c00085
Jahani, P. M., Beitollahi, H., & Tajik, S. (2022). Surface amplification of graphite screen printed electrode using reduced graphene oxide/polypyrrole nanotubes nanocomposite; a powerful electrochemical strategy for determination of sulfite in food samples. Food Chem Toxicol, 167, 113274. doi.org/10.1016/j.fct.2022.113274
Joint, F. A. O. W. H. O. E. C. o. F. A. (2009). Evaluation of Certain Food Additives. Retrieved from https://apps.who.int/iris/handle/10665/43979
Karuwan, C., Wisitsoraat, A., Chaisuwan, P., Nacapricha, D., & Tuantranont, A. (2017). Screen-printed graphene-based electrochemical sensors for a microfluidic device [10.1039/C7AY00379J]. Analytical Methods, 9(24), 3689-3695. doi.org/10.1039/C7AY00379J
Kim, H. J. (1990). Determination of sulfite in foods and beverages by ion exclusion chromatography with electrochemical detection: collaborative study. J Assoc Off Anal Chem, 73(2), 216-222.
Kim, H. J., & Kim, Y. K. (2006). Analysis of Free and Total Sulfites in Food by Ion Chromatography with Electrochemical Detection. Journal of Food Science, 51, 1360-1361. doi.org/10.1111/j.1365-2621.1986.tb13122.x
Koch, M., Köppen, R., Siegel, D., Witt, A., & Nehls, I. (2010). Determination of Total Sulfite in Wine by Ion Chromatography after In-Sample Oxidation. Journal of Agricultural and Food Chemistry, 58(17), 9463-9467. doi.org/10.1021/jf102086x
Lee, D., Ro, H., Hwang, S., Lee, M., Kim, H., Heo, J., & Cha, S. (2024). Determination of Sulfites in Dried Fruits by Paper Spray Ionization Tandem Mass Spectrometry. Molecules, 29(10). doi.org/10.3390/molecules29102192
Lee, H. C., Liu, W.-W., Chai, S.-P., Mohamed, A. R., Aziz, A., Khe, C.-S., Hidayah, N. M. S., & Hashim, U. (2017). Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Advances, 7(26), 15644-15693. doi.org/10.1039/C7RA00392G
Lozer, T., Prezilius, A., dos Santos, G., Schaffel, I., Ramon Rosa, T., & Ferreira, R. (2022). ®®Development of a portable electroanalytical methodology for determination of sulfite in wine using screen-printed carbon electrodes modified with carbon nanotubes. Journal of Food Composition and Analysis, 116, 105052. doi.org/10.1016/j.jfca.2022.105052
Martins, P. R., Popolim, W. D., Nagato, L. A. F., Takemoto, E., Araki, K., Toma, H. E., Angnes, L., & Penteado, M. D. V. C. (2011). Fast and reliable analyses of sulphite in fruit juices using a supramolecular amperometric detector encompassing in flow gas diffusion unit. Food Chemistry, 127(1), 249-255. doi.org/10.1016/j.foodchem.2010.12.103
Mercy Magomya, A., Garbunga Yebpella, G., Chidinma Okpaegbe, U., John Oko, O., & Bature Gambo, S. (2020). Analysis and Health Risk Assessment of Sodium Benzoate and Potassium Sorbate in Selected Fruit Juice and Soft Drink Brands in Nigeria. International Journal of Pharmacy and Chemistry, 6(5). doi.org/10.11648/j.ijpc.20200605.11
Ministry of Public Health, & Food and Drug Administration. (2000). Ministry of Public Health Announcement No. 214 (2000) on Food Additives. Retrieved from https://food.fda.moph.go.th/law/data/announ_moph/214-43.pdf
Mohammadzadeh Jahani, P., Beitollahi, H., Tajik, S., Aflatoonian, M. R., Garkani Nejad, F., Zaimbashi, R., & Mohammadnavaz, A. (2024). CeO2 nanoparticles modified screen-printed carbon electrode: Electrochemical sensing platform for sulfite determination in water samples. International Journal of Electrochemical Science, 19(7). doi.org/10.1016/j.ijoes.2024.100621
Molinero-Abad, B., Alonso-Lomillo, M. A., Domínguez-Renedo, O., & Arcos, J. (2013). Amperometric determination of sulfite using screen-printed electrodes modified with metallic nanoparticles. Microchimica Acta, 180, 1351-1355. doi.org/10.1007/s00604-013-1074-8
Pasakon, P., Primpray, V., Thangphatthanarungruang, J., Kamsong, W., Wisitsoraat, A., Laiwattanapaisal, W., Intasanta, V., & Karuwan, C. (2024). Conductive disposable screen-printed graphene oxide-molybdenum disulfide electrode for electrochemical sensing applications. Electrochemistry Communications, 166.doi.org/10.1016/j.elecom.2024.107778
Pungjunun, K., Yakoh, A., Chaiyo, S., Siangproh, W., Praphairaksit, N., & Chailapakul, O. (2022). Smartphone-based electrochemical analysis integrated with NFC system for the voltammetric detection of heavy metals using a screen-printed graphene electrode. Mikrochim Acta, 189(5), 191. doi.org/10.1007/s00604-022-05281-x
Sartori, E., Takeda, H., & Fatibello-Filho, O. (2011). Glassy Carbon Electrode Modified with Functionalized Carbon Nanotubes Within a Poly(allylamine hydrochloride) Film for the Voltammetric Determination of Sulfite in Foods. Electroanalysis, 23. doi.org/10.1002/elan.201100122
Shaha, A., Esrafil, M., Akter, S., Bari, L., Khan, M. S. H., Alam, M. J., Dina, P. R., & Zubair, M. A. (2024). Determination of sodium benzoate in different brands of orange juices available in Bangladesh by high-performance liquid chromatography. Food Research, 8(1), 313-318. doi.org/10.26656/fr.2017.8(1).198
Silva, E. M., Takeuchi, R. M., & Santos, A. L. (2015). Carbon nanotubes for voltammetric determination of sulphite in some beverages. Food Chem, 173, 763-769. doi.org/10.1016/j.foodchem.2014.10.106
Verma, S. K., & Deb, M. K. (2007). Single-Drop and Nanogram Level Determination of Sulfite (SO32−) in Alcoholic and Nonalcoholic Beverage Samples Based on Diffuse Reflectance Fourier Transform Infrared Spectroscopic (DRS-FTIR) Analysis on KBr Matrix. Journal of Agricultural and Food Chemistry, 55(21), 8319-8324. doi.org/10.1021/jf071344c
Winiarski, J. P., de Barros, M., Magosso, H., & Jost, C. (2017). Electrochemical reduction of sulfite based on gold nanoparticles/silsesquioxane-modified electrode. Electrochimica Acta, 251. doi.org/10.1016/j.electacta.2017.08.171
Zhang, X., Liao, X., Wang, Y., Rao, L., & Zhao, L. (2024). Health effects of fruit juices and beverages with varying degrees of processing. Food Science and Human Wellness, 13(5), 2456-2479. doi.org/10.26599/fshw.2022.9250202
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



