First Report of Cytogenetics of Whipfin Mojarra (Gerres filamentosus, Cuvier 1829) from Pattani, Thailand
Keywords:
chromosome, karyotype, Gerres filamentosus , marine fishAbstract
Background and Objectives : The whipfin silver-biddy (Gerres filamentosus) is a marine fish that is important both ecologically and economically. It belongs to the order Perciformes and the suborder Percoidei, It is classified under the family Gerridae, which comprises 53 species of bony fishes (Froese & Pauly, Eds., n.d.).A key characteristic of this species is its body shape, which appears stout, resembling an oval or a flattened rhombus. It has a small, short head, large eyes, a pointed snout, and a protrusible mouth. The scales are small and easily shed. In mature individuals, the second hard ray of the dorsal fin extends into a single long filament, and dark-colored spots are arranged in 7–10 horizontal rows along the body, which distinguishes it from other fish in the same group. The whipfin silver-biddy is considered an indicator species of the richness of food chains in areas with high biological productivity (Ramírez-Luna et al., 2008). It is also a food source for larger marine animals. Additionally, the foraging behavior of this fish along the seabed promotes nutrient and oxygen cycling within the ecosystem. It prefers to inhabit tropical to temperate marine environments (Randall, 1995). In Thailand, this species has been reported in Chumphon, Prachuap Khiri Khan, Samut Prakan, Chonburi, and Rayong provinces (Tanpitayakup, 2013), with a high density along the coastal areas of Pattani Bay (Chuapun et al., 2017; Hajisamae et al., 2006). Recently, cytotaxonomy methods are being applied by utilizing knowledge of cell genetics to classify fish species (Esmaeili et al., 2008). Additionally, this helps in understanding evolutionary relationships and genetic changes that have occurred in the past. Understanding the chromosomal structure of marine fish helps in planning the conservation of endangered species by enabling the assessment of genetic diversity and preventing the loss of this diversity (Kasi-ruek, 2014). It is also beneficial for the development of breeding economically important fish species and the study of molecular biology to sustainably conserve marine resources (Arai, 2011). Currently, cytogenetic information on fish in the genus Gerres and the family Gerreidae remains limited, and there have been no reports of chromosomal studies on the whipfin silver-biddy (Gerres filamentosus).This study aims to examine the chromosome characteristics and identify chromosome markers using conventional chromosome staining and NOR-banding techniques to locate ribosomal RNA (rRNA) gene sites. The techniques used in the study can help visualize each chromosome pair and clearly show the unique characteristics of certain chromosomes (Campiranon, 2003). The information obtained will be useful for studying taxonomic relationships and enhancing understanding of evolutionary processes. It may also benefit the management and conservation of this species in the future.
Methodology : The study of chromosomes of Gerres filamentosus was conducted using the direct method.The kidney was used as the organ of choice since it has continuous cell division (Campiranon, 2003). The chromosome preparation using the direct method in vivo was adapted from the methods of Chen & Ebeling (1968), Nanda et al. (1995). Chromosome were prepared by injecting a 0.05% colchicine solution at a volume of 1 milliliter/100 grams of body weight. Kidney tissue continuous cell division was then soaked in a potassium chloride (KCl) solution before being centrifuged. The cells obtained were dropped onto clean slides, 2–3 drops, with each drop placed 2 centimeters apart. Allow them to air dry stained using conventional banding and NOR-banding. The slides were examined under a compound microscope. Chromosome analysis involves selecting cells with well-spread metaphase chromosomes that are not overlapping. The chromosomes are then photographed using a 100X objective lens.
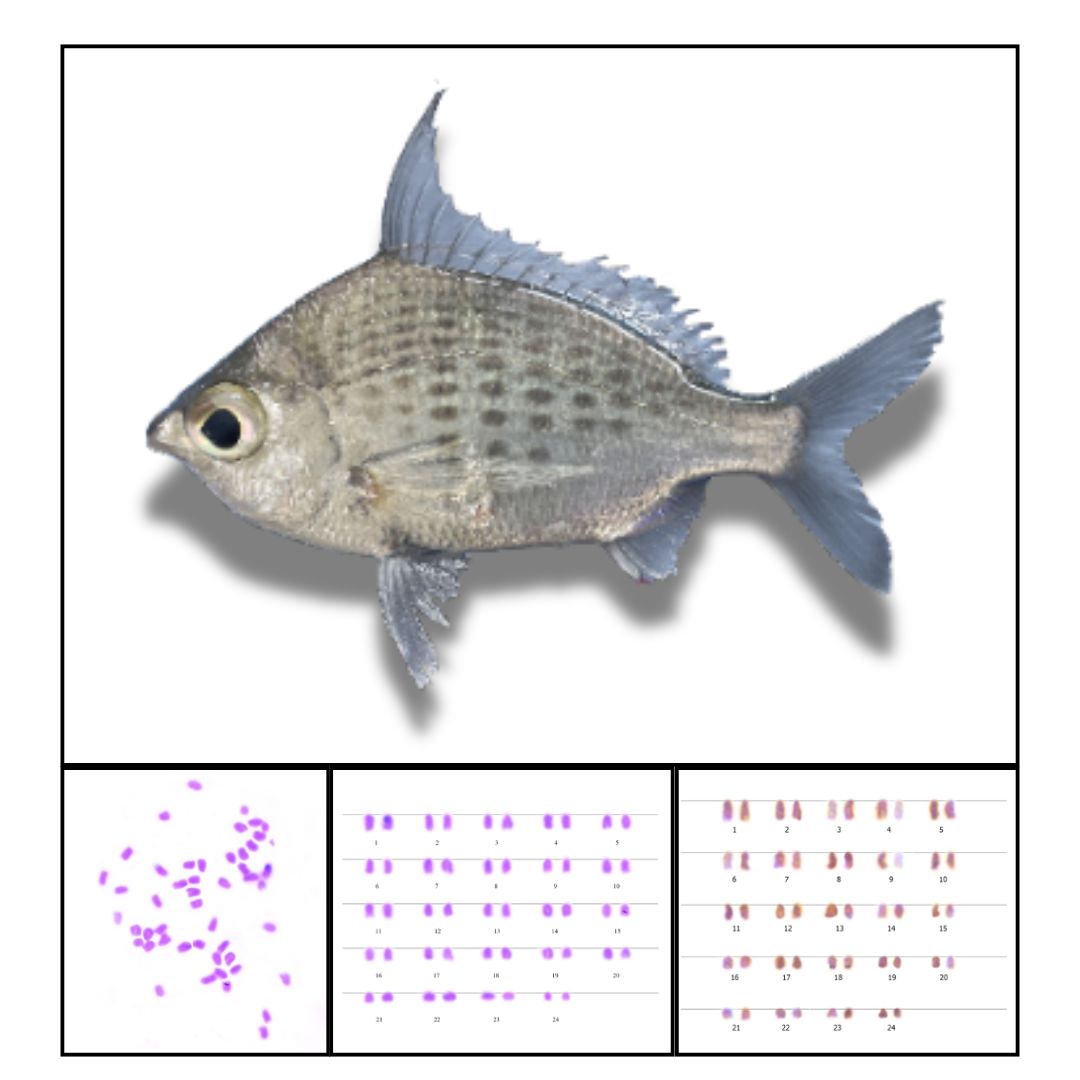
Main Results : The karyotype study of Whippin Silver-Biddy (G. filamentosus) revealed a diploid chromosome number (2n) of 48 for both male and female specimens. The fundamental number (NF) is also 48. The karyotype consists of 48 telocentric chromosomes. Using NOR banding, one pair of NORs (nucleolus organizer region) was identified on the telomere region of the long arm of the medium telocentric chromosome pair 18 (18 q tel)
Conclusions : From this study, it was found that the whipfin silver-biddy (Gerres filamentosus) has a diploid chromosome number of 2n = 48. The fundamental number is also 48, consisting of 30 large telocentric chromosomes, 12 medium-sized telocentric chromosomes, and 6 small telocentric chromosomes. A single pair of nucleolar organizer regions (NORs) was observed near the telomeric region on the long arm of the medium-sized telocentric chromosome pair 18. No karyotypic differences were found between male and female individuals. These findings indicate that the whipfin silver-biddy has the same chromosome number as other previously studied species in the family Gerreidae (8 pecies), with all showing a single NOR location but at different chromosomal positions. This suggests that the chromosome number is consistent with that of most marine fishes in the order Perciformes (Kasi-ruek, 2014). This study represents the first report on cytogenetic and NOR localization in G. filamentosus. It not only enhances our understanding of the chromosomal structure of this species but also provides essential baseline information for future genetic studies and conservation planning. The karyotype formula of G. filamentosus is as follows: 2n (48) = L30t + M12t+ S6t (1)
References
Affonso, P.R.A.d.M., & Galetti, P.M. (2005). Chromosomal diversification of reef fishes from genus Centropyge (Perciformes, Pomacanthidae). Genetica ,123, 227-233.
Allen, G. R. (1991). Field guide to the freshwater fishes of New Guinea.
Allen, G.R., Midgley, S.H., & Allen, M. (2002). Field guide to the freshwater fishes of Australia. Western Australian Museum, Perth, Western Australia. 394.
American Veterinary Medical Association (AVMA). (2020). AVMA Guidelines for the Euthanasia of Animals. 2020Edition. Retrieved from https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf
Arai, R. (2011). Fish karyotypes: a check list. Springer Science & Business Media. doi.org/10.1007/978-4-431-53877-6
Asha, P. S., Joshi, K. K., & Diwakar, K. (2009). Incidence of fish mortality in Tuticorin Bay, Gulf of Mannar. Journal of the Marine Biological Association of India, 51(2), 173-177.
Brum, N. J. (1995). Cytogenetic studies of Brazilian marine fishes. Brazilian Journal of Genetics, 18(3), 421-427.
Calado, L.L., Bertollo, L.A.C., Costa, G.W.W.F.D, & Molina, W.F. (2012). Cytogenetic studies of Atlantic mojarras (Perciformes – Gerreidae): chromosomal mapping of 5S and 18S ribosomal genes using double FISH. Aquaculture Research, 44, 829–835.
Calado, L. L., Bertollo, L. A. C., Cioffi, M. B., Costa, G. W. W. F., Jacobina, U. P., & Molina, W. F. (2014).Evolutionary dynamics of rDNA genes on chromosomes of the Eucinostomus fishes: cytotaxonomic and karyoevolutive implications. Genetics and Molecular Research, 13(4), 9951-9959.
Campiranon, A. (2003). Cytogenetics. (2). Bangkok: Kasetsart University Press.
Carpenter, K. E. (1997). Living marine resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates. Food & Agriculture Organization
Carpenter, K. E., & Niem, V. H. (Eds.). (2001). FAO species identification guide for fishery purposes: The living marine resources of the Western Central Pacific. Volume 5. Bony fishes part 3 (Menidae to Pomacentridae), 2791–3380. Food and Agriculture Organization.
Chen, T. R., & Ebeling, A. W. (1968). Karyological evidence of female heterogamety in the mosquitofish, Gambusia affinis. Copeia, 70-75.
Chuapun, K., Augsornpa-ob, U., Sanitmajjaro, W., & Pankaew, K. (2017). Marine Resources in 10 Nautical-mile Inshore Area of the Gulf of Thailand. Technical Paper No.7/2017. Marine Fisheries Research and Development Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. 10-28. (in Thai)
Eschmeyer, W. N., Fricke, R., Fong, J. D., & Polack, D. A. (2010). Marine fish diversity: history of knowledge and discovery (Pisces). Zootaxa, 2525(1), 19-50.
Esmaeili, H. R., Ebrahimi, M., & Saifali, M. (2008). Karyological analysis of five tooth-carps (Actinopterygii: Cyprinodontidae) from Iran. Micron, 39(2), 95-100.
Froese, R., & Pauly, D. (Eds.). (n.d.). Species in genus Gerres. FishBase. Retrieved from https://www.fishbase.org/identification/SpeciesList.php?genus=Gerres.
Galetti, P. M., Aguilar, C. T., & Molina, W. F. (2000). An overview of marine fish cytogenetics. Marine genetics, 55-62.
Hajisamae, S., Yeesin, P., & Chayamongkol, S. (2006). Habitat utilization by fishes in a shallow, semi-enclosed estuarine bay in the southern Gulf of Thailand. Estuarine, Coastal and Shelf Science. 68, 647-655.
Jantarat, S., Jumrusthanasan, S., Kaewsri, S., Supanuam, P., & Tanomtong, A. (2021). First report of karyological analysis and heteromorphic nucleolar organizer region of Black Surgeonfish (Acanthurus gahhm, Acanthuridae) in Thailand. Caryologia, 74(1), 83-88.
Kasi-ruek, W. (2014). Cytogenetics of Mandarin Fish (Synchiropus sp.). Institute of Marine Science, Burapha University, 16-29. (in Thai)
Larson, H., Dahanukar, N., Molur, S., & Sparks, J.S. ( 2017). Gerres filamentosus. The IUCN Red List of Threatened Species 2017: e.T166897A46643777. doi.org/10.2305/IUCN.UK.2017-3.RLTS.T166897A46643777.en
Letourneur, Y., Kulbicki, M., & Labrosse, P. (1998). Length-weight relationships of fish from coral reefs and lagoons of New Caledonia, southwestern Pacific Ocean: an update. Naga, 21(4),39-46.
Marine and Coastal Resources Research & Development Institute. (2021). Estuaries fishes of inner Gulf of Thailand. Bangkok, Thailand: Department of Marine and Coastal Resources, A.P. Printing Media Co., Ltd. 140. (in Thai)
Nanda, I. M. W. I. J. M., Schartl, M., Feichtinger, W., Schlupp, I., Parzefall, J., & Schmid, M. (1995). Chromosomal evidence for laboratory synthesis of a triploid hybrid between the gynogenetic teleost Poecilia formosa and its host species. Journal of Fish Biology, 47(4), 619-623.
Ozouf-Costaz, C., & Foresti, F. (1992). Fish cytogenetics. Oceanography and Marine Biology: An Annual Review, 30, 263-314.
Paim, F. G., de Oliveira Nobile, M. L. M., Foresti, F., & Oliveira, C. (2018). Cytogenetic tools to study the biodiversity of Neotropical fish: From the classic to the advent of cell culture. In Cytogenetics-Past, Present and Further Perspectives. IntechOpen. doi.org/10.5772/intechopen.80332
Ramírez-Luna, V. I. V. I. A. N. A., Navia, A. F., & Rubio, E. A. (2008). Food habits and feeding ecology of an estuarine fish assemblage of northern Pacific Coast of Ecuador. Pan-American Journal of Aquatic Sciences, 3(3), 361-372.
Randall, J.E., (1995). Coastal fishes of Oman. University of Hawaii Press, Honolulu, Hawaii. 439.
Ruiz-Carus, R., & Uribe-Alcocer, M. (2003). Karyotype analysis of Eucinostomus argenteus, E. gula, E. harengulus, and Eugerres plumieri (Teleostei, Gerreidae) from Florida and Puerto Rico. Environmental biology of fishes, 67, 269-276.
Sosa-Nishizaki, O., De la Rosa-Velez, J., & Grijalva-Chon, J. M. (1996). A1lozyme variability in two samples of swordfish, Xiphias gladius L., in the North Pacific Ocean. Fishery bulletin, 94(3), 589-594.
Tanomtong, A. (2010). Cytogenetics. Khon Kaen University Press. Khon Kaen. 299-319. (in Thai)
Tanomthong, A., & Pinthong, K. (2019). Cytogenetics. (1). Chulalongkorn University. (in Thai)
Tanpitayakup, C., Charuthanin, K., & Chotisuwan, N. (2013). Amphidromous story: The ultimate predator, two-water fish, "Wild Ambition". Aquarium Biz, 3(35), 110–129. May 2013. (in Thai)
Van Der Laan, R., Eschmeyer, W. N., & Fricke, R. (2014). Family-group names of recent fishes. Zootaxa, 3882(1), 1-230.
Woodland, D. J. (1984). Gerreidae. In W. Fischer & G. Bianchi (Eds.), FAO species identification sheets for fishery purposes: Western Indian Ocean fishing area, 51(2).
Woodland, D.J., (1986). Siganidae. In M.M. Smith and P.C. Heemstra (eds.) Smiths' sea fishes. Springer-Verlag, Berlin. 824-825.
Wright, J.M., (1988). Seasonal and spacial differences in the fish assemblage of the non-estuarine Sulaibikhat Bay, Kuwait. Marine Biology, 100, 13-20.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



