Simple Synthesis of Benzothiazole and Benzothiazoline Compounds Using Cyanuric Chloride as Catalyst
คำสำคัญ:
simple synthesis , benzothiazole, benzothiazoline, cyanuric chloride , 2-aminothiophenolบทคัดย่อ
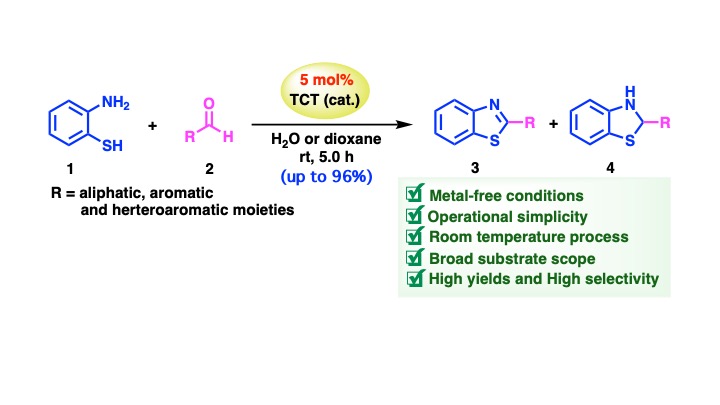
Background and Objectives: The synthesis of benzothiazole and benzothiazoline derivatives, crucial heterocyclic compounds widely applied in pharmaceuticals and materials science has attracted increasing interest due to their diverse biological and chemical properties. These compounds exhibit antibacterial, anticancer, and antioxidant activities, as well as important optical and electronic features, making them valuable for various applications in medical and industrial fields. Traditional synthetic routes for these compounds, however, often involve toxic reagents, expensive catalysts, and harsh reaction conditions that generate significant chemical waste and pose environmental and safety risks. For instance, the use of metal-based catalysts and hazardous solvents in conventional methods increases the environmental footprint and costs of production. As a result, there is a pressing need to develop alternative, sustainable, and environmentally friendly approaches for the synthesis of these compounds. Therefore, this study aims to develop a green and efficient synthetic approach for the synthesis of benzothiazole and benzothiazoline derivatives. Cyanuric chloride, a non-toxic, low-cost catalyst, was selected as a highly efficient catalyst. The use of water, an environmentally benign solvent was explored as a reaction medium, in comparison to organic solvents. The influence of various aldehyde substrates, particularly those bearing electron-donating and electron-withdrawing groups, on the yield of the desired products was also investigated. This study explores the optimization of reaction parameters and the application of green chemistry principles in heterocyclic synthesis, with the goal of achieving high yields of the target compounds under mild conditions.
Methodology: The synthetic approach employed in this study involved the reaction of 2-aminothiophenol with a range of aldehydes, including aliphatic, aromatic, and heterocyclic aldehydes, in a 1:1 molar ratio. Cyanuric chloride was used as the catalyst for the Friedel-Crafts cyclocondensation reaction. A systematic investigation was conducted to optimize the reaction such as catalyst loading, type and volume of solvent, reaction time, and reaction temperature. The optimization process involved the reactions under mild conditions, typically at room temperature. Both water, as a green solvent, and a variety of organic solvents with different polarities were evaluated. Water was chosen for its low environmental impact, as it is non-toxic, abundant, and inexpensive. Several organic solvents were investigated, including polar protic solvents such as methanol and ethanol, polar aprotic solvents such as tetrahydrofuran, dimethylformamide, and 1,4-dioxane, non-polar solvents such as 1,2-dichloroethane, acetonitrile, and toluene as well as an ionic liquid ([bmim][Br]). The reactions were monitored using thin-layer chromatography (TLC), and the product was confirmed by nuclear magnetic resonance (NMR) spectroscopy.
Main Results: The reaction successfully yielded both benzothiazole and benzothiazoline derivatives in yields ranging from 22% to 98%, depending on the aldehyde type and solvent used. Notably, aldehydes with electron-donating groups such as methyl and methoxy substituted aromatic aldehydes led to higher yields of benzothiazole derivatives. On the other hand, aldehydes with electron-withdrawing groups such as nitro or carbonyl functional groups tended to favor the formation of benzothiazolines, albeit in moderate yields. These results suggest that electron-donating groups enhance the nucleophilicity of the aldehydes, thereby facilitating the cyclocondensation reaction. A key finding of this study was that water, when used as the solvent, performed comparably or even better than conventional organic solvents, demonstrating its viability as a green solvent for this reaction. In some cases, water even provided higher yields of the desired products. This result supports the concept of green chemistry, where water can be used as a sustainable alternative to toxic and expensive organic solvents. The reactions proceeded smoothly at room temperature, and no additional heating or the use of hazardous reagents was required, further enhancing the eco-friendly nature of the approach. Catalytic efficiency was also evaluated using various catalysts, including cyanuric chloride, dodecyl benzenesulfonic acid (DBSA), and iron(III) chloride hexahydrate (FeCl3·6H2O). The study confirmed that cyanuric chloride was the most efficient catalyst for this reaction, affording higher yields of the desired products. In contrast, control reactions conducted without any catalyst produced significantly lower yields, indicating the essential role of cyanuric chloride in facilitating the cyclocondensation. The reaction time and solvent volume were identified as crucial factors in determining the yield and stability of the products. Prolonging the reaction time to 6.0 hours resulted in reduced yields and signs of product degradation, while shortening the reaction time to 1.0 hour led to incomplete conversion of the reactants. Similarly, reducing the water volume to 1.0 mL led to lower yields, likely due to an increase in the acidity of the reaction medium, which negatively impacted the reaction efficiency. These findings emphasize the importance of optimizing reaction conditions for achieving high yields of the desired products.
Conclusions: This study demonstrates that cyanuric chloride (TCT) is an effective and efficient catalyst for the synthesis of benzothiazole and benzothiazoline derivatives through Friedel-Crafts cyclocondensation of 2-aminothiophenol with a variety of aldehydes. The reaction was successfully optimized under mild conditions, with both water and 1,4-dioxane as optimal solvents. The reaction proceeded smoothly at room temperature, thus confirming the eco-friendly nature of the approach. The developed protocol, which utilizes cyanuric chloride and water as a catalyst and solvent, respectively, aligns with the principles of green chemistry, including the reduction of chemical waste, low energy consumption, and minimized environmental impact. The substrate scope analysis indicated that the method is broadly applicable to a wide range of aldehydes, with aromatic aldehydes, particularly those with electron-donating substituents, leading to high yields of benzothiazole derivatives. In contrast, aldehydes with electron-withdrawing groups favored the formation of benzothiazolines. This study not only provides a sustainable, low-cost, and safe method for the synthesis of benzothiazole and benzothiazoline derivatives but also contributes to the development of green synthetic routes in organic chemistry. The findings of this research provide a strong foundation for future work on sustainable heterocyclic synthesis and offer a promising avenue for large-scale industrial applications, where both safety and environmental impact are critical considerations.
เอกสารอ้างอิง
Al-Qalaf, F., Mekheimer, R. A., & Sadek, K. U., (2008). Cerium (IV) ammonium nitrate (CAN) catalyzed one-pot synthesis of 2-arylbenzothiazoles. Molecules, 13(11), 2908-2914.
Ali, I., Fozia, B., Syeda, A. Z. N., Amjad, I., Sameh, M. O., Alessio N., Siham, A. A., & Claudiu, T. S. (2020). Benzothiazole derivatives as anticancer agents. Journal of Enzyme Inhibition and Medicinal Chemistry, 35(1), 265-279.
Asiri, Y., Alsayari, A., Muhsinah, A. B., Mabkhot, Y. N., & Hassan, M. Z. (2020). Benzothiazoles as potential antiviral agents. Journal of Pharmacy and Pharmacology, 72(11), 1459-1480.
Bigdeli, M. A., Heravi, M. M. , & Mahdavinia, G. H. (2007). Wet cyanuric chloride catalyzed simple and efficient synthesis of 14-aryl or alkyl-14-H-dibenzo [a,j] xanthenes. Catalysis Communications. 8(11), 1595–1598.
Blunt, C.E. Nawrat, C.C. LeBozec, L. Liutkus, M. Liu, Y. Lewis, W., & Moody, C.J. (2016). Oxidative routes to the heterocyclic cores of benzothiazole natural products. Synlett, 27(1), 37-40.
Chaudhari, C., Siddiki S. M. A. H. , & Shimizu, K. (2015). Acceptorless dehydrogenative synthesis of benzothiazoles and benzimidazoles from alcohols or aldehydes by heterogeneous Pt catalysts under neutral conditions. Tetrahedron Letters, 56(34), 4885-4888.
Chhabra, M., Sinha S., Banerjee S., & Paira, P. (2016). An efficient green synthesis of 2- arylbenzothiazole analogues as potent antibacterial and anticancer agents. Bioorganic & Medicinal Chemistry Letters. 26(1), 213-217.
Fan, L.-Y., Shang, Y.-H., Li, X.-X., & Hua, W.-J. (2015). Yttrium-catalyzed heterocyclic formation via aerobic oxygenation: A green approach to benzothiazoles. Chinese Chemical Letters, 26(1), 77-80.
Fazaeli, R., & Aliyan, H. (2009). A heterogeneous catalyst for efficient and green synthesis of 2-arylbenzothiazoles and 2-arylbenzimidazoles. Applied Catalysis A : General, 353(1), 74–79.
Ghafuri, H., Esmaili, E., & Talebi, M. (2016). Fe3O4@SiO2/collagen: An efficient magnetic nanocatalyst for the synthesis of benzimidazole and benzothiazole derivatives. Comptes Rendus Chimie, 19(8), 942–950. doi.org/10.1016/j.crci.2016.05.003
Guo, H. Y., Li, J. C., & Shang, Y. L, (2009). A simple and efficient synthesis of 2-substituted benzothiazoles catalyzed by H2O2/HCl. Chinese Chemical Letters, 20(12), 1408-1410.
Gupta, A., & Rawat, S. (2010). Synthesis and cyclization of benzothiazole : Review. Journal of Current Pharmaceutical Research, 3(1), 13-23.
Gupta, K., Sirbaiya, A. K., Kumar, V., & Rahman, M. A. (2022). Current perspective of synthesis of medicinally relevant benzothiazole based molecules: Potential for antimicrobial and anti-Inflammatory Activities. Mini-Reviews in Medicinal Chemistry, 22(14, 1895-1935.
Inamdar, S. M. More V. K., & Mandal, S. K. (2013). CuO nano-particles supported on silica, a new catalyst for facile synthesis of benzimidazoles, benzothiazoles and benzoxazoles. Tetrahedron Letters, 54(6), 579-583. doi.org/10.1016/j.tetlet.2012.11.091
Ji, S.-J., & Shi, H.-B. (2006). Synthesis and fluorescent property of some novel benzothiazoyl pyrazoline derivatives containing aromatic heterocycle. Dyes and Pigments, 70(3), 246-250.
Kashyap, P., Verma, S., Gupta, P., Narang, R., Lal, S., & Devgun, M. (2023). Recent insights into antibacterial potential of benzothiazole derivatives. Medicinal Chemistry Research, 32, 1543-1573.
Khokra, S. L. Arora, K., Mehta, H., Aggarwal, A., & Yadav, M. (2011). Common Methods to synthesize benzolthiazole derivatives and their medicinal significance. International pharmaceutical sciences and research. 2(6), 1356-1377.
Kim, Y., Kumar, M. R. Park, N., Heo, Y., & Lee, S., (2011). Copper-catalyzed, one-pot, three-component synthesis of benzimidazoles by condensation and C-N bond formation. Organic chemistry, 76(23), 9577–9583.
Kumar, G., & Singh, N. P. (2021). Synthesis, anti-inflammatory and analgesic evaluation of thiazole/oxazole substituted benzothiazole derivatives, Bioorganic Chemistry, 107, 104608.
Kumar, V., Gupta, G. K., & Kumar, M. (2011). PTSA catalyzed solid phase synthesis of some 2-arylbenzothiazoles an expeditious, mild and greener protocol. Current Trends in Biotechnology and Chemical, 1(2), 2249-4073.
Lee, A. S., Chung, C. Chang, Y., & Chen P., (2012). L-proline catalyzed condensation reaction of aldehyde or carboxylic acid with 2-aminothiophenol under solvent-Free and microwave irradiation. Journal of Applied Science and Engineering, 15(3), 311-315.
Li, W. L., Luo, Q. Y. ,& Yan, F. L. (2011). Cyanuric chloride-catalyzed synthesis of 10-aryl-6,8-dimethyl-6,10-dihydro-5-oxa-6,8-diazaanthra[2,3-d][1,3]dioxole-7,9-diones. Chinese Chemical Letters, 22(7), 811-814.
Mahdavinia, G. H. , & Bigdeli, M. A. (2009). Wet cyanuric chloride promoted efficient synthesis of amidoalkyl naphthols under solvent-free conditions. Chinese Chemical Letters. 20(4), 383-386.
Maleki, B., Azarifar, D. Veisi, H., Hojati, S. F., Salehabadi, H., & Yami, R. N. (2010). Wet 2,4,6-trichloro-1,3,5-triazine (TCT) as an efficient catalyst for the synthesis of 2,4,6-triarylpyridines under solvent-free conditions. Chinese Chemical Letters, 21(11), 1346-1349.
Marsilje, T. H., Hedrick, M.P. Desharnais, J., Tarassoli, A., Zang, Y., Wilson, T. A., Benkonic, S.J., & Boger, D. L. (2003). Design, synthesis, and biological evaluation of simplified α-keto heterocycle trifluoromethyl ketone, and formyl substituted folate analogues as potential inhibitors of GAR transformylase and AICAR transformylase. Bioorganic & Medicinal Chemistry, 11(20), 4487-4501.
Mayo, M. S., Yu, X. Zhou, X., Feng, X., Yamamoto, Y., & Bao, M. (2014). Convenient synthesis of benzothiazoles and benzimidazoles through Brønsted acid catalyzed cyclization of 2-amino thiophenols/anilines with β-diketones. Organic letters, 16(3), 764-767. doi.org/10.1021/ol403475v
Meghdadi, S., Amirnasr, M., & Ford P. C. (2012). A robust one-pot synthesis of benzothiazoles from carboxylic acids including examples with hydroxyl and amino substituents. Tetrahedron Letters, 53(51), 6950-6953. doi.org/10.1016/j.tetlet.2012.10.035
Miron, T., & Wilchek, M. (2017). A sensitive colorimetric determination of cyanuric chloride and its activated agarose immobilization resins. Analytical Biochemistry, 527(15), 1-3.
Pasha, M. A., & Nizam, A. (2012). Amberlite IR-120 catalyzed, microwave-assisted rapid synthesis of 2-aryl-benzimidazoles. Journal of Saudi Chemical Society, 16(3), 237-240.
Riadi, Y., Azzalou, R. M. R. Haddad, M. E., Routier, S., Guillaumet, G., & Lazar, S. (2011). An efficient and reusable heterogeneous catalyst animal bone meal for facile synthesis of benzimidazoles, benzoxazoles, and benzothiazoles. Tetrahedron Letters, 52(27), 3492-3495.
Rodionov, V.O., Presolski, S.I., Gardinier, S., Lim, Y.H., & Finn, M.G. (2007). Benzimidazole and related ligands for Cu-catalyzed azide-alkyne cycloaddition. Journal of the American Chemical Society, 129(42), 12696-12704.
Senapak, W., Saeeng, R., Jaratjaroonphong, J., & Sirion, U. (2018). Bronsted acid-surfactant-combined ionic liquid catalyzed green synthesis of 2-alkyl and 2-arylbenzothiazoles in water: Reusable catalyst and metal-free conditions. Molecular Catalysis, 458, 97-105.
Siddalingamurthy, E. Mahadevan, K. M., Masagal, J. N., & Harishkumar, H. N. (2013). Mild efficient Fischer indole synthesis using 2,4,6-trichloro-1,3,5-triazine (TCT). Tetrahedron Letters, 54(41), 5591-5596.
Sivaguru, P., Theerthagiri, P., & Lalitha, A. (2015). Cyanuric chloride catalyzed synthesis of 5-substituted-1H-tetrazoles. Metal free organic transformation, 56(17), 2203–2206.
ดาวน์โหลด
เผยแพร่แล้ว
รูปแบบการอ้างอิง
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2025 คณะวิทยาศาสตร์ มหาวิทยาลัยบูรพา

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



