Protein Digestibility of Ingredients in Banana Shrimp Fenneropenaeus merguiensis (De Man, 1888)
Keywords:
Banana Shrimp feed , protein hydrolysate , shrimp feed ingredients , trypsinAbstract
Background and Objectives : Banana Shrimp (Fenneropenaeus merguiensis) is one of the economically important domestic species of marine shrimp in Thailand. Banana Shrimp culture has not been succeeded since the lack of basic knowledge, including the specific shrimp feed for Banana Shrimp has not been successfully formulated. This study aimed to determine the protein digestibility of shrimp feed raw materials using in vitro technique with crude enzyme extract of shrimp larvae from protozoeal 1 (PZ1) to postlarval 60 (PL60) stages, in order to establish the data base for Banana Shrimp feed development and formulate the efficient shrimp feeds, which meet the shrimp nutrition requirements for growth and stage development, with the highest feed utilization and the least of waste to reduce the environmental problems, as well as cost saving and cost effectiveness of formulated diet for successfully and sustainably Banana Shrimp culture in the near future.
Methodology : Banana Shrimp gravid females from the wild were transferred to the hatchery in Chonburi Aquatic Animal Feed Research and Development Center. Shrimp larvae were hatched and nursed and larval samples were collected at protozoeal (PZ) 1-3, mysis (M) 1-3, postlarval (PL) 1, 20, 30, 40, 50 and 60 stages and then stored at -80 °C until analyzed. Then crude enzyme was extracted using 50 mM Tris-HCl buffer, pH 8, then the supernatant was collected and stored at -80 °C for further analysis. There were 10 feed ingredients tested: - Artemia, soybean meal, soybean meal hydrolysate, shrimp head meal, squid meal, fish meal, krill, yeast, Spirulina and marine fish waste protein hydrolysate. For in vitro digestibility, Trinitrobenzene sulphonic acid (TNBS) was determined in the feed ingredients which were digested with crude enzyme from Banana Shrimp, compared to DL-alanine standard curve as µmol DL-alanine per g feed per trypsin specific activity. Trypsin specific activity (µmol p-Nitroaniline) was measured using 1.25 mM bensoyl-L-arginine-p-nitroanilide (BAPNA) as substrate and p-Nitroaniline as a standard. For total protein was determined followed the method of Bradford and bovine serum albumin (BSA) was used as a standard. The protein digestibility of feed ingredient data were subjected to Analysis of variance (One-way ANOVA) and Duncan's New Multiple Range test at 95% confidence interval (p < 0.05).
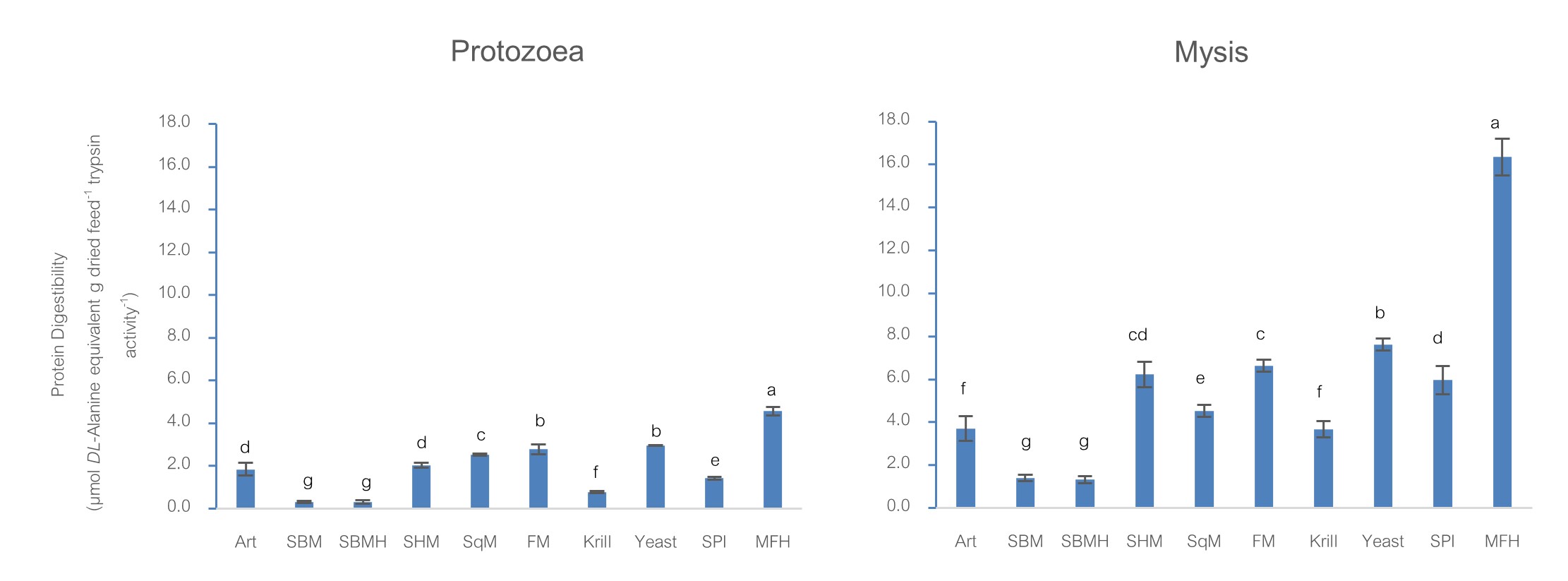
Main Results : It was found that for all stages of Banana Shrimp larvae (PZ1- PL60), the significantly (p<0.05) highest protein digestibility was marine fish waste protein hydrolysate (MFH). The following ranks differed among the stages of shrimp. On the other hand, soybean meal and soybean meal hydrolysate had the significantly (p<0.05) lowest digestibility in all stages (PZ1- PL60) of Banana Shrimp. When compared the protein digestibility of MFH and fish meal, which is the main protein raw material from animal in shrimp feed, the digestibility of MFH was 1.64-2.85 times higher than those of fish meal in every stage of tested Banana shrimp larva. It was even higher when compared to the protein digestibility of soybean meal, which is the main protein raw material from plant in shrimp feed, the digestibility of MFH was 11-22 times and 45-67 times higher than those of soybean meal in PZ1 to PL1 and PL20-60 stages, respectively. For trypsin activity of Banana Shrimp larvae, it was peaked at PZ2 and slightly declined in PZ3, but the average trypsin in PZ stages were higher than those of Mysis and the later stages. The activity dropped, when the larvae developed into M1 stage, and continued decreasing through M2 and M3. When the larvae metamorphosed into PL1, trypsin activity increased again. As the activity defined per protein unit, therefore, the bigger the size of larvae, the smaller the trypsin activity from PL20-PL60 stages.
Conclusions : Banana Shrimp, from PZ1 to PL60 stages, had the highest efficiency for MFH protein digestibility. The following ranks differed among the stages of shrimp. Yeast, fish meal, squid meal and Spirulina were in high digestible protein ingredients group, followed by shrimp head meal, krill and Artemia. While the digestibility efficiency of hydrolyzed soybean meal and soybean meal of the PZ1 to PL60 stages of the shrimp was the lowest. MFH- marine fish waste protein hydrolysate has the potential to be used as an alternative shrimp feed ingredient, especially to replace fish meal at an appropriate level. It is a valuable use of waste, reduces waste, and increases nutritional value. For the specific trypsin activity in Banana Shrimp, it showed the suitable feeding for juvenile shrimp in each stage: - protozoa stage feeding on phytoplankton (herbivores), mysis stage feeding on both phytoplankton and zooplankton (omnivores) and postlarval stage feeding on zooplankton (carnivores), respectively. From the results of the efficiency of protein digestibility of shrimp feed raw materials and trypsin activity in Banana Shrimp from PZ1-PL60 stages could be the guidelines for formulating the feed formula for Banana Shrimp culture in the future.
References
Bradford, M.M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248-254. doi.org/10.1016/0003-2697(76)90527-3.
Buathong, T., Foowut, J., & Krongpong, L. (2019). Potential of protein hydrolysate production from marine fish waste with 3 selected bacteria strains. In Proceeding Annual Conference on Fisheries 2019. (pp. 55-66.) Bangkok: Department of Fisheries. (in Thai)
Chaklader, M.R., Howieson, J., Foysal, M.J., Hanif, M.A., Abdel-Latif, H.M.R., & Fotedar, R. (2023). Fish waste to sustainable additives: Fish protein hydrolysates alleviate intestinal dysbiosis and muscle atrophy induced by poultry by-product meal in Lates calcarifer juvenile. Frontiers in Nutrition, 10, 1145068. doi.org/10.3389/fnut.2023.1145068.
Chungyampin, S., & Phumchuai, C. (2000). Culture of banana shrimp, Penaeus merguiensis De Man, for broodstock in earthen ponds. No.24/2000. Trang Coastal Aquaculture Station, Coastal Aquaculture Division, Department of Fisheries,18 p. (in Thai)
Department of Fisheries. (2024a). FISHERIES STATISTICS OF THAILAND 2023. No. 11/2024. Fishery Statistics Group, Fisheries Development Policy and Planning Division, Department of Fisheries, Thailand, 93 p. (in Thai)
Department of Fisheries. (2024b). STATISTICS OF MARINE SHRIMP CULTURE 2023. No. 10/2024. Fishery Statistics Group, Fisheries Development Policy and Planning Division, Department of Fisheries, Thailand, 44 p. (in Thai)
Domínguez, H., Iñarra, B., Labidi, J., & Bald, C. (2024). Fish viscera hydrolysates and their use as biostimulants for plants as an approach towards a Circular Economy in Europe: A Review. Sustainability, 16(20), 8779. doi.org/10.3390/su16208779.
Ezquerra, J.M., García Carreño, F.L., Civera, R., & Haard, N. (1997). pH-stat method to predict protein digestibility in white shrimp (Penaeus vannamei). Aquaculture, 157, 251-262. doi.org/10.1016/S0044-8486(97)00058-6.
Fan, Z., Wu, D., Li, C. Zhou, M., Wang, L., Zhang, H., Li, J., Rong, X., Miao, L., Zhao, D., & Wang, J. (2024). Application of fish protein hydrolysates in common carp (Cyprinus carpio) diets for fish meal sparing: Evidence from growth, intestinal health and microflora composition. Aquaculture Reports, 36, 102160. doi.org/10.1016/j.aqrep.2024.102160.
Fernández Gimenez, A. V., Díaz, A. C., Velurtas, S. M., & Fenucci, J. L. (2009). In vivo and in vitro protein digestibility of formulated feeds for Artemesia longinaris (Crustacea, Penaeidae). Brazilian Archives of Biology and Technology, 52(6), 1379-1386. doi.org/10.1590/S1516-89132009000600009.
Iadnoi, Y. Ingkhakul, A., Kovitwathi, U., & Thankitjanukit, S. (2007). Characterization and in vitro digestibility of digestive enzyme in giant black tiger shrimp Penaeus monodon, white shrimp Penaeus vannamei and giant fresh water prawn Macrobrachium rosenbergii. Journal of Fisheries Technology Research, 1(2), 248-260. (in Thai)
Jones, D.A., Kumlu, M., Le Vay, L., & Fletcher, D.J. (1997). The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: a review. Aquaculture, 155(1–4), 285-295.doi.org/10.1016/S0044-8486(97)00129-4.
Kanazawa, A. (1989). Microparticulate feeds for Penaeid larvae. Advances in tropical aquaculture,pp.395-404.
Khosravi, S., Bui, H.T.D., Rahimnejad, S., Herault, M., Fournier, V., Jeong, J.B., & Lee, K.-J. (2015).Effect of dietary hydrolysate supplementation on growth performance, non-specific immune response and disease resistance of olive flounder (Paralichthys olivaceus) challenged with Edwardsiella tarda. Aquaculture nutrition, 21(3), 321-331. doi.org/10.1111/anu.12157.
Klompong, V., Benjakul, S., Kantachote, D., & Shahidi, F. (2007). Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selariodes leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food chemistry, 102(4), 1317-1327.doi.org/10.1016/j.foodchem.2006.07.016.
Kolkovski, S., & Tandler, A. (2000). The use of squid protein hydrolysate as a protein source in microdiets for gilthead seabream Sparus aurata larvae. Aquaculture Nutrition, 6(1), 11-15.doi.org/10.1046/j.1365-2095.2000.00125.x.
Kumlu, M. (1995). Physiology of decapod crustacean larvae with special reference to diet. (Ph.D. Thesis). School of Ocean Science, University of Wales, Bangor, UK.
Lee, P.G., & Lawrence, A.L. (1997). Digestibility. Advances in World Aquaculture, 6, 194-260.
Lemos, D., Garcia-Carreño, F.L., Hernandez, P., & Navarrete del Toro, A. (2002). Ontogenetic variation in digestive proteinase activity, RNA and DNA content of larval and post larval white shrimp Litopenaeus schmitti. Aquaculture, 214, 363-380. doi.org/10.1016/S0044-8486(02)00253-3.
Lemos, D., Hernandez-Cortes, M. P., Navarrete, A., García-Carreño, F., & Phan, V. N. (1999). Ontogenetic variation in digestive proteinase activity of larvae and postlarvae of the pink shrimp Farfantepenaeus paulensis (Crustacea: Decapoda: Penaeidae). Marine Biology, 135(4), 653–662.doi.org/10.1007/S002270050666.
Le Vay, L., Rodríguez, A., Kamarudin, M.S., & Jones, D.A. (1993). Influence of live and artificial diets on tissue composition and trypsin activity in Penaeus japonicus larvae. Aquaculture, 118(3-4), 287-297. doi.org/10.1016/0044-8486(93)90463-9.
Maytorena-Verdugo, C. I., Córdova-Murueta, J. H., & García-Carreño, F. (2017). Peptidase compensation in the digestive system of white leg shrimp Penaeus vannamei against dietary Kunitz‐type soybean trypsin inhibitor. Aquaculture Nutrition, 23(5), 1095–1103. doi.org/10.1111/ANU.12477.
Nguyen, H. T. M., Perez-Galvez, R. , & Berge, J. P. (2012). Effect of diets containing tuna head hydrolysate on the survival and growth of shrimp Penaeus vannamei. Aquaculture, 324-325, 127-134. doi.org/10.1016/j.aquaculture.2011.11.014.
Nguyen, M.C., Fotedar, R., & Giridharan, B. (2023). The effects of fish protein hydrolysate as supplementation on growth performance, feed utilization and immunological response in fish: A review. MATEC web of conferences. doi.org/10.1051/matecconf/202337701020.
Okedigba, A. O., Gómez Rosso, L., Yu, D., Shang, C., Huang, H., Zhang, B., & Capelluto, D. G. S. (2023). Comparative binding affinity analysis of soybean meal Bowman-Birk and Kunitz trypsin inhibitors in interactions with animal serine proteases. ACS Food Science & Technology, 3(8), 1344–1352. doi.org/10.1021/acsfoodscitech.3c00158.
Ovissipour, R., Abedian Kenari, A., Nazari, R., Motamedzadegan, A., & Rasco, B. (2014). Tuna viscera protein hydrolysate: nutritive and disease resistance properties for Persian sturgeon (Acipenser persicus L.) larvae. Aquaculture research, 45(4), 591-601. doi.org/10.1111/j.1365-2109.2012.03257.x.
Pongmaneerat, J., Chainark, P., & Chindamaikul, T. (2003). Protein digestibility of some feed ingredients in diets black tiger shrimp (Penaeus monodon Fabricius) and banana prawn (Penaeus merguiensis). No.15/2003. Phangnga Coastal Fisheries Rearch and Development Center, Department of Fisheries, 22 p. (in Thai)
Puello-Cruz, A. C., Sangha, R. S., Jones, D. A., & Le Vay, L. (2002). Trypsin enzyme activity during larval development of Litopenaeus vannamei (Boone) fed on live feeds. Aquaculture Research, 33(5), 333-338. doi.org/10.1046/j.1365-2109.2002.00676.x.
Rungruangsak-Torrissen, K., Rustad, A., Sunde, J., Eiane, S.A., Jensen, H.B., Opstvedt, J., Nygard, E., Samuelsen, T.A., Mundheim, H., Luzzana, U., & Venturini, G. (2002). In vitro digestibility based on fish crude enzyme extract for prediction of feed quality in growth trials. Journal of the Science of Food and Agriculture, 82, 644-654. doi.org/10.1002/jsfa.1089.
Rungruangsak-Torrissen, K., Moss, R., Andresen, L.H., & Waagbø, R. (2006). Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiology and Biochemistry, 32, 7–23. doi.org/10.1007/s10695-005-0630-5.
Sanchez-Paz, A., Garcia-Carreno, L.F., Muhlia-Almazan, A., Hernandez-Saavedra, N., & Yepiz-Plascencia, G. (2003). Differential expression of trypsin mRNA in the white shrimp Penaeus vannamei midgut gland under starvation conditions. Journal of Experimental Marine Biology and Ecology, 292, 1-7. doi.org/10.1016/S0022-0981(03)00142-4.
Sandbakken, I.S., Johansen L., Zhang, Y., Ringø, E., Røsbak, R., Yakovlev, I., Five, K., & Olsen, R.E. (2024). Replacing fishmeal with salmon hydrolysate reduces the expression of intestinal inflammatory markers and modulates the gut microbiota in Atlantic salmon (Salmo salar). Frontiers in Marine Science, 11, 1376516. doi.org/10.3389/fmars.2024.1376516.
Siddik, S., Howieson, J., Partridge, G., Fotedar, R., & Gholipourkanani, H. (2018). Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Scientific Reports, 8(1), 15942-13. doi.org/10.1038/s41598-018-34182-4.
Tacon, A.G.J., & Metian, M. (2008). Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture, 285, 146-158. doi.org/10.1016/j.aquaculture.2008.08.015.
Thongprajukaew, K., & Kovitvadhi, U. (2012). Enzymes and Food Development for Aquaculture. The Journal of King Mongkut’s University of Technology North Bangkok, 22(3), 710-720. (in Thai)
Turkmen, G., & Baysal, S. H. (2007). Trypsin Enzyme Activity during Larval Development of Penaeus semisulcatus De Haan, 1844, Fed on Live Feeds. Crustaceana, 80(2), 225–234. Retrieved from http://www.jstor.org/stable/20107798.
Wu, H. C., Chen, H. M., & Shiau, C. Y. (2003). Free amino acid and peptide as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Research International, 36, 949-957. doi.org/10.1016/S0963-9969(03)00104-2.
Xu, H., Mu, Y., Zhang, Y., Li, J., Liang, M., Zheng, K., & Wei, Y. (2016). Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): effects on growth performance and lipid accumulation. Aquaculture, 454, 140-147. doi.org/10.1016/j.aquaculture.2015.12.006.
Zheng, K., Xu, T., Qian, C., Liang, M., & Wang, X. (2014). Effect of low molecular weight fish protein hydrolysate on growth performance and IGF-I expression in Japanese flounder (Paralichthys olivaceus) fed high plant protein diets. Aquaculture nutrition, 20(4), 372-380. doi.org/10.1111/anu.12090.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



