Selection of Probiotic Bacterium Capable of GABA Production for the Application as Feed Supplement in Laying Hens
Keywords:
probiotics, GABA, laying hens , heat stress , feed supplementsAbstract
Background and Objectives : Main problems affecting the raising of laying hens in Thailand, especially on the growth and efficiency of egg production are the epidemics and heat stress. Supplementation of viable probiotic cells to poultry diet has been widely accepted as an effective treatment in the reduction of infection and promoting poultry health instead of the use of antibiotics treatment. Nowadays, the antibiotics supplementation in the livestock production is restricted and controlled in many countries including Thailand. In addition, the gamma – aminobutyric acid (GABA) substance plays a significant role in mitigation of heat stress impacts on growth and egg production efficiency of laying hens. Thus, the discovery of GABA - producing probiotic strain, a dual - functional feed supplement, with high specificity to poultry gastrointestinal tract is a promising strategy for the laying hens raising and egg production. The objectives of this research were then to screen the probiotic strains capable of GABA production from the digestive tract of laying hens for the application in feed supplement purposes, especially reduction of the impacts from above mentioned problems and to investigate the survivability of the probiotic cells after the formulation of product powder.
Methodology : Isolation of lactic acid bacteria from the cloaca of laying hens was conducted on de Man Rogosa and Sharpe (MRS) agar. Those isolates were subjected to the preliminary in vitro tests of their probiotic properties. The tests included the investigation of hemolytic activity (alpha -, beta - and gamma - hemolysis) on sheep blood agar, the growth ability under the acid conditions (pH 2.0, 2.5, 3.0 and 3.5) and the presence of bile (0.3 and 1.0% (w/v)), the cell surface hydrophobicity (%CSH) assessed by the auto-aggregation ability of cells after toluene addition, the antibiotic susceptibility on 13 antibiotics interpreted according to the standard protocol of the Clinical and Laboratory Standards Institute (CLSI), and the antimicrobial activity against some enteric pathogens; i.e. Escherichia coli, Salmonella Typhi and Staphylococcus aureus, using the spot on lawn technique. Subsequently, the ability of the selected isolates on GABA production in MRS broth supplemented by 0.5% (w/v) monosodium glutamate was studied. GABA was quantitatively and qualitatively determined by using spectrophotometry method and thin layer chromatography (TLC), respectively. Finally, species identification of the selected isolate by the analysis of 16S rRNA gene nucleotide sequencing and used to query GenBank via BLAST was investigated. Furthermore, probiotic powder was formulated by mixing with rice bran and drying by freeze-drying process for further study of the cell survivability when stored at 4oC and room temperature for 12 weeks in the sealed aluminum foil bag.
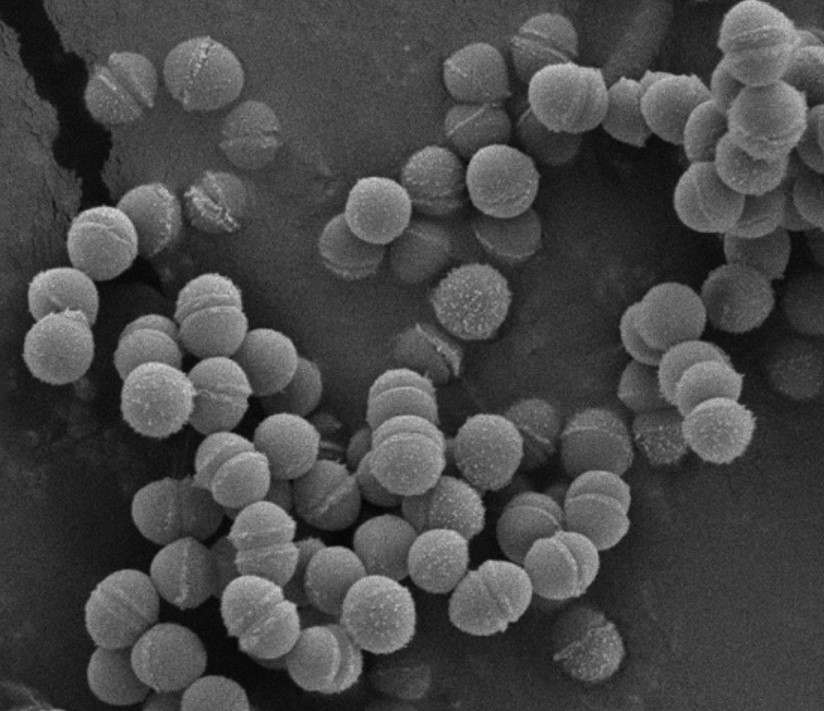
Main Results : The results showed that 42 isolates of lactic acid bacteria were obtained from the feces samples collected from laying hens cloaca. They were Gram-positive bacteria, non-catalase producing bacteria and capable of acid production. Moreover, it was found that their morphology of cell shapes were coccus and rod (5 cocci- and 37 rod-shape isolates). After the preliminary test of probiotic properties, it was investigated that isolate LH5, a coccus shape bacterium that occurs in pairs and short chains, exhibited the highest potential of probiotic properties when tested under the simulated conditions of poultry digestive tract. It could grow at all tested pH levels (2.0–3.5) and high bile concentration of 1.0% (w/v). Its cell surface hydrophobicity was at the moderate level with the cell surface hydrophobicity value of 57%. The isolate LH5 was susceptible to penicillin, ampicillin, vancomycin, cefoxitin, chloramphenicol, clindamycin, ceftriaxone, ciprofloxacin, cephalothin and rifampicin, while resisted to three antibiotics, i.e. gentamycin, erythromycin and nalidixic acid. This isolate possessed the antimicrobial activity against two pathogenic bacteria, i.e. S. Typhi, a harmful pathogen causing Salmonellosis outbreak in poultry, and S. aureus. The isolate LH5 did not show a hemolytic activity on sheep blood agar (gamma-hemolysis). Subsequently, the isolate LH5 was identified as Enterococcus faecium with the similarity of 100%. It could also produce GABA of 39.05 mg/L and GABA could be obviously detected on silica gel - coated aluminum sheet with high intensity. In addition, the formulation of probiotic powder was achieved. The survival rate of E. faecium LH5 in probiotic powder was high at 95.39 + 0.07 and 94.15 + 0.19 % after the 12 weeks of storage at 4oC and room temperature (approximately 30oC), respectively, and not significantly different (p>0.05) comparing between both temperatures at each week.
Conclusions : Enterococcus faecium LH5 bacterium was a potential GABA producing probiotic. Its cells were stable during the formulation process into a powder form and the storage at room temperature. Therefore, it was a promising probiotic strain for further utilization as the feed additive in laying hens. However, a study on the effect of E. faecium LH5 supplementation on growth performances and the efficiency of egg production under the challenge of either heat stress or pathogenic infection is necessary to be further investigated before applying as a feed supplement.
References
Abdallah, F. B., Lagha, R., Khaled, S., Kallel, H., & Gharbi, J. (2014). Detection of cell surface hydrophobicity, biofilm and fimbirae genes in Salmonella isolated from Tunisian clinical and poultry meat. Iranian Journal of Public Health, 43(4), 423.
Ben-Miled, H., Benoit-Biancamano, M.-O., Ben-Mahrez, K., & Réjiba, S. (2023). Alpha-amylase and alphaglucosidase inhibitory properties, beta-galactosidase activity, and probiotic potential of lactic acid bacteria and bifidobacteria from Apis mellifera intermissa and its products. World Journal of Microbiology and Biotechnology, 39(8), 205.
Bouchard, D. S., Seridan, B., Saraoui, T., Rault, L., Germon, P., Gonzalez-Moreno, C., Nader-Macias, F. M., Baud, D., François, P., & Chuat, V. (2015). Lactic acid bacteria isolated from bovine mammary microbiota: potential allies against bovine mastitis. PloS one, 10(12), e0144831.
Bs, S., Thankappan, B., Mahendran, R., Muthusamy, G., Femil Selta, D. R., & Angayarkanni, J. (2021). Evaluation of GABA production and probiotic activities of Enterococcus faecium BS5. Probiotics and Antimicrobial Proteins, 13, 993-1004.
Carvajal, E., Contreras, S., Díaz, W., Martinez-Bello, D., McCown, M., Ardila, Y., & Vásquez, M. C. (2023). Enterococcus isolated from poultry intestine for potential probiotic use. Veterinary World, 16(8), 1605.
Casertano, M., Fryganas, C., Valentino, V., Troise, A.D., Vitaglione, P., Fogliano, V., & Ercolini, D. (2024). Gut production of GABA by a probiotic formula: an in vitro study. Beneficial Microbes, 15(1), 67-81.
Chantanawilas, P., Pahumunto, N., & Teanpaisan, R. (2024). Aggregation and adhesion ability of various probiotic strains and Candida species: an in vitro study. Journal of Dental Sciences,19(4), 2163-2171.
Chen, Z., Niu, C., Wei, L., Huang, Z., & Ran, S. (2024). Genome-wide analysis of acid tolerance genes of Enterococcus faecalis with RNA-seq and Tn-seq. BMC Genomics, 25, 261.
Cruz, A. G., Antunes, A. E., Sousa, A. L. O., Faria, J. A., & Saad, S. M. (2009). Ice-cream as a probiotic food carrier. Food Research International, 42(9), 1233-1239.
Devi, P. B., Rajapuram, D. R., Jayamanohar, J., Verma, M., Kavitake, D., Avany, B. A. M., Rani, P. U., Ravi, R., & Shetty, P. H. (2023). Gamma-aminobutyric acid (GABA) production by potential probiotic strains of indigenous fermented foods origin and RSM based production optimization. LWT, 176, 114511.
Diana, M., Quílez, J., & Rafecas, M. (2014). Gamma-aminobutyric acid as a bioactive compound in foods: a review. Journal of Functional Foods, 10, 407-420.
Elkomy, A., Zahra, A.E., Belih, S., Shaheen, Y., & Aboubakr, M. (2020). Effect of drinking water supplemented with gamma-aminobutyric acid (GABA) on laying performance and egg quality parameters in laying hens. International Journal of Advanced Research, 8, 312-317
Elhussiny, M. Z., Tran, P. V., Pham, C. V., Nguyen, L. T., Haraguchi, S., Gilbert, E. R., Cline, M. A., Bungo, T., Furuse, M., & Chowdhury, V. S. (2021). Central GABAA receptor mediates taurine-induced hypothermia and possibly reduces food intake in thermo-neutral chicks and regulates plasma metabolites in heat-exposed chicks. Journal of Thermal Biology, 98, 102905.
Frank, J.A., Reich, C.I., Sharma, S., Weisbaum, J.S., Wilson, B.A., & Olsen, G.J. (2008). Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Applied and Environmental Microbiology, 74, 2461–2470.
Gibson, G.R., Probert, H.M., Loo, J.V., Rastall, R.A., & Roberfroid, M.B. (2004). Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutrition Research Reviews, 17, 259–275.
Gil, M. G., Gomez-Raya, L., Torres, O., Cigarroa-Vazquez, F. A., Davila, S. G., & Rauw, W. M. (2023). Heterophil/lymphocyte response of local Spanish breeds of laying hens to cold stress, heat stress, and water restriction. Journal of Thermal Biology, 113, 103542.
Gul, K., Yousuf, B., Singh, A., Singh, P., & Wani, A. A. (2015). Rice bran: Nutritional values and its emerging potential for development of functional food - A review. Bioactive Carbohydrates and Dietary Fibre, 6(1), 24-30.
Gunn, J. S. (2000). Mechanisms of bacterial resistance and response to bile. Microbes and Infection, 2(8), 907-913.
Han, J., Zhao, X., Zhao, X., Wang, Q., Li, P., & Gu, Q. (2023). Microbial-derived -aminobutyric acid: synthesis, purification, physiological function, and applications. Journal of Agricultural and Food Chemistry, 71(41), 14931-14946.
Hervo, F., Létourneau-Montminy, M. P., Méda, B., & Narcy, A. (2024). Effect of limestone particle size and microbial phytase on phosphorus and calcium digestion kinetics along the gastrointestinal tract in laying hens. British Poultry Science, 65(5), 605–614.
Huang, S., Rong, X., Liu, M., Liang, Z., Geng, Y., Wang, X., Zhang, J., Ji, C., Zhao, L., & Ma, Q. (2022).Intestinal mucosal immunity-mediated modulation of the gut microbiome by oral delivery of Enterococcus faecium against Salmonella Enteritidis pathogenesis in a laying hen model.Front Immunol., 2022 (13), 853954.
Jeong, S.-B., Kim, Y. B., Lee, J.-W., Kim, D.-H., Moon, B.-H., Chang, H.-H., Choi, Y.-H., & Lee, K.-W. (2020). Role of dietary gamma-aminobutyric acid in broiler chickens raised under high stocking density. Animal Nutrition, 6(3), 293-304.
Jo, M.-H., Hong, S.-J., Lee, H.-N., Ju, J.-H., Park, B.-R., Lee, J.-h., Kim, S.-A., Eun, J.-B., Wee, Y.-J., & Kim,Y.-M. (2019). Gamma-aminobutyric acid production from a novel Enterococcus avium JS-N6B4 strain isolated from edible insects. Poultry Science, 95(12), 2829-2835.
Kalode, V., & Patil, P. (2023). Enterococcus species: a systemic review. Journal of Pure & Applied Microbiology, 17(2).
Khalifa, A., & Ibrahim, H. I. M. (2023). Enterococcus faecium from chicken feces improves chicken immune response and alleviates Salmonella infections: A pilot study. Journal of Animal Science, 101, skad016.
Kierończyk, B., Rawski, M., Długosz, J., Świąkiewicz, S., & Józefiak, D. (2016). Avian crop function–a review. Annals of Animal Science, 16(3), 653-678.
Kim, D.-H., Dasagrandhi, C., Park, S.-K., Eom, S.-H., Huh, M.-K., Mok, J.-S., & Kim, Y.-M. (2018). Optimization of gamma-aminobutyric acid production using sea tangle extract by lactic acid bacterial fermentation. LWT, 90, 636-642.
Kim, D.-H., & Lee, K.-W. (2023). An update on heat stress in laying hens. World's Poultry Science Journal, 79(4), 689-712.
Kim, Y. B., Park, J., Lee, H.-G., Song, J.-Y., Kim, D.-H., Ji, W., Joo, S. S., Kim, M., Jung, J. Y., & Kim, M. (2024). Dietary probiotic Lacticaseibacillus paracasei NSMJ56 modulates gut immunity and microbiota in laying hens. Poultry Science, 103505.
Kolupaev, Y. E., Shakhov, I., Kokorev, A., Dyachenko, A., & Dmitriev, A. (2024). The role of reactive oxygen species and calcium ions in implementing the stress-protective effect of -aminobutyric acid on wheat seedlings under heat stress conditions. Cytology and Genetics, 58(2), 81-91.
Li, T., Luo, B., Zou, X., Yin, J., Lv, S., Wang, J., & Gong, L. (2024). Enterococcus avium from a traditional Chinese liquor fermentation system with the potential for the de novo synthesis of GABA. LWT, 206, 116560.
Mangan, M., & Siwek, M. (2024). Strategies to combat heat stress in poultry production - A review. Journal of Animal Physiology and Animal Nutrition, 108(3), 576-595.
Martínez, S., López, M., & Bernardo, A. (2003). Thermal inactivation of Enterococcus faecium: effect of growth temperature and physiological state of microbial cells. Letters in Applied Microbiology, 37(6), 475-481.
Maasjost J., Mühldorfer K., De Jäckel S.C., & Hafez H.M. (2015). Antimicrobial susceptibility patterns of Enterococcus faecalis and Enterococcus faecium isolated from poultry flocks in Germany. Avian Diseases, 59, 143–148.
Morgan, N., Walk, C., Bedford, M., & Burton, E. (2014). The effect of dietary calcium inclusion on broiler gastrointestinal pH: Quantification and method optimization. Poultry Science, 93(2), 354-363.
Park, J., Jeong, J., Lee, S., & Kim, I. (2016). Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poultry Science, 95(12), 2829-2835.
Park, J., & Kim, I. (2015). Effects of dietary gamma-aminobutyric acid on egg production, egg quality, and blood profiles in layer hens. Veterinarni Medicina, 60(11), 629-634.
Phuengjayaem, S., Booncharoen, A., & Tanasupawat, S. (2021). Characterization and comparative genomic analysis of gamma-aminobutyric acid (GABA)-producing lactic acid bacteria from Thai fermented foods. Biotechnology Letters, 43(8), 1637-1648.
Recoules, E., Lessire, M., Labas, V., Duclos, M. J., Combes-Soia, L., Lardic, L., Peyronnet, C., Quinsac, A., Narcy, A., & Réhault-Godbert, S. (2019). Digestion dynamics in broilers fed rapeseed meal. Scientific Reports, 9(1), 3052.
Rodríguez-Sánchez, S., Fernández-Pacheco, P., Seseña, S., Pintado, C., & Palop, M. L. (2021). Selection of probiotic Lactobacillus strains with antimicrobial activity to be used as biocontrol agents in food industry. LWT, 143, 111142.
Ruiz, L., Margolles, A., & Sánchez, B. (2013). Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Frontiers in Microbiology, 4, 396.
Salim, H. M., Huque, K. S., Kamaruddin, K. M., & Beg, M. (2018). Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Science Progress, 101(1), 52-75.
Shaji, S., Selvaraj, R. K., & Shanmugasundaram, R. (2023). Salmonella infection in poultry: a review on the pathogen and control strategies. Microorganisms, 11(11), 2814.
Sharma, C., Gulati, S., Thakur, N., Singh, B. P., Gupta, S., Kaur, S., Mishra, S. K., Puniya, A. K., Gill, J. P. S., & Panwar, H. (2017). Antibiotic sensitivity pattern of indigenous lactobacilli isolated from curd and human milk samples. 3 Biotech, 7, 1-14.
Sirisopapong, M., Shimosato, T., Okrathok, S., & Khempaka, S. (2023). Assessment of lactic acid bacteria isolated from the chicken digestive tract for potential use as poultry probiotics. Animal Bioscience, 36(8), 1209.
Sobczak, A., & Kozłowski, K. (2015). The effect of a probiotic preparation containing Bacillus subtilis ATCC PTA-6737 on egg production and physiological parameters of laying hens. Annals of Animal Science, 15(3), 711-723.
Špelina, V., Schlemmerová, L., Landfeld, A., Kýhos, K., Měřička, P., & Houška, M. (2007). Thermal inactivation of Enterococcus faecium. Czech Journal of Food Sciences, 25(5), 283.
Tamura, K., Stecher, G., & Kumar, S. (2021). MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38(7), 3022-3027.
Tan, X., Zhang, Q., Liu, J., Shang, Y., Min, Y., Sun, X., & Tang, J. (2024). Enhanced -aminobutyric acid production by co-culture fermentation with Enterococcus faecium AB157 and Saccharomyces cerevisiae SC125. LWT, 208, 116739.
Tancharoenrat, P., Zaefarian, F., & Ravindran, V. (2022). Composition of chicken gallbladder bile. British Poultry Science, 63(4), 548-551.
Xu, Y., Li, D., Ding, X., Wang, Y., Liang, S., Xie, L., Zhang, Y., Fu, A., Yu, W., & Zhan, X. (2023). Probiotic characterization and comparison of broiler-derived lactobacillus strains based on technique for order preference by similarity to ideal solution analysis. Poultry Science, 102(5), 102564.
Yang, B., Huang, S., Zhao, G., & Ma, Q. (2022). Dietary supplementation of porcine bile acids improves laying performance, serum lipid metabolism and cecal microbiota in late-phase laying hens. Animal Nutrition, 11, 283-292.
Yao, L., Lyu, C., Wang, Y., Hu, S., Zhao, W., Cao, H., Huang, J., & Mei, L. (2023). High-level production of -aminobutyric acid via efficient co-expression of the key genes of glutamate decarboxylase system in Escherichia coli. Engineering Microbiology, 3, 100077.
Zhang, M., ZOU, X. t., Li, H., DONG, X. y., & Zhao, W. (2012). Effect of dietary ‐aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat‐stressed Roman hens. Animal Science Journal, 83(2), 141-147.
Zhang, Q., Xiang, J., Zhang, L., Zhu, X., Evers, J., Werf, W., & Duan, L. 2014. Optimizing soaking and germination conditions to improve gamma-aminobutyric acid content in japonica and indica germinated brown rice. Journal of Functional Foods ,10, 283-291.
Zhou, X., Willems, R. J., Friedrich, A. W., Rossen, J. W., & Bathoorn, E. (2020). Enterococcus faecium: from microbiological insights to practical recommendations for infection control and diagnostics. Antimicrobial Resistance & Infection Control, 9, 1-13.
Zhu, Y., Cheng, J., Ren, M., Yin, L., & Piao, X. (2015). Effect of -aminobutyric acid-producing Lactobacillus strain on laying performance, egg quality and serum enzyme activity in Hy-Line brown hens under heat stress. Asian-Australasian Journal of Animal Sciences, 28(7), 1006.
Zommiti, M., Chevalier, S., Feuilloley, M.G.J., & Connil, N. (2022). Special Issue "Enterococci for probiotic use: safety and risk": Editorial. Microorganisms, 10(3), 604.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Faculty of Science, Burapha University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Burapha Science Journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) licence, unless otherwise stated. Please read our Policies page for more information



